Home Size: 1 2 3 4 5 6 7 8 9
|
 |
| The non-metal on which all living chemistry depends. Carbon exists in multiple forms: diamond, graphite, amorphous and fullerenes.
|
|
|
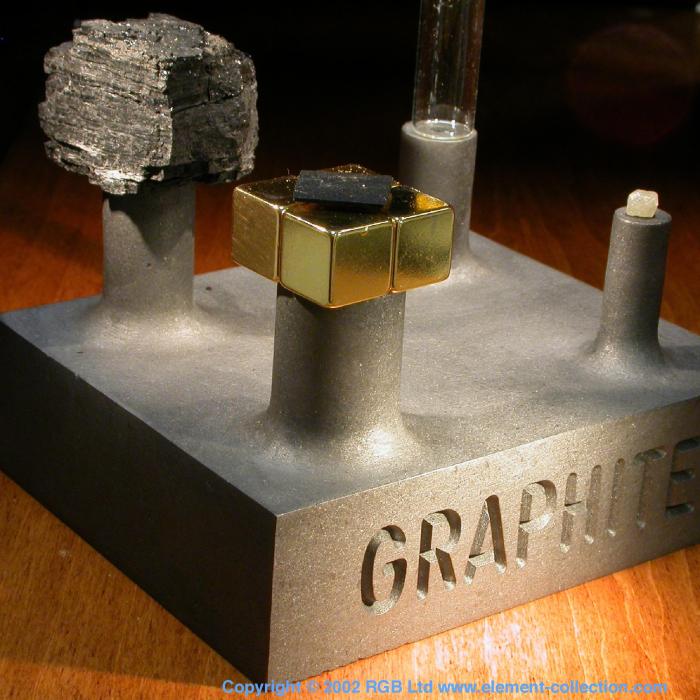 |
Machined graphite block.
This plinth is machined from a solid block of graphite. It is an excellent material to mill being easily cut but capable of taking fine detail. All the dust does tend to make a mess of the shop however.
Source: eBay
Size: 5"
Purity: 99.9%
|
|
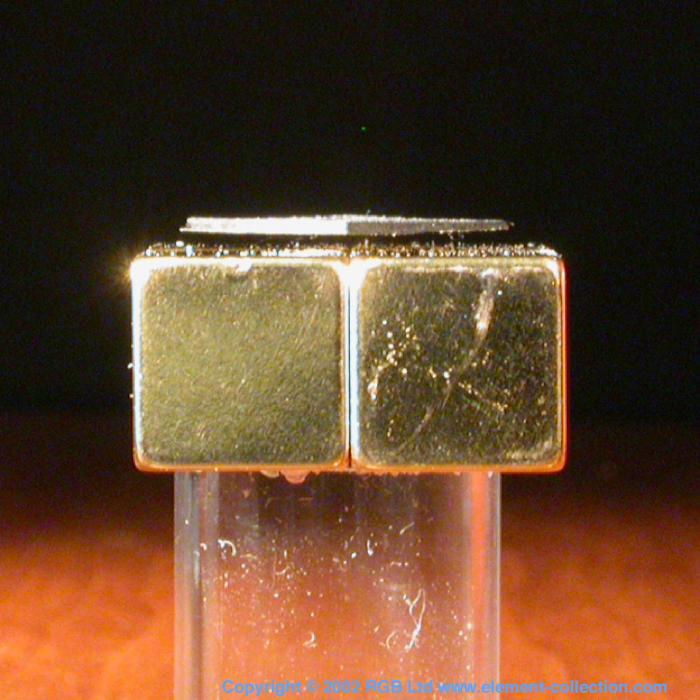 |
Levitating pyrolytic graphite.
The carbon here is the thin square of graphite floating above the golden magnets. There are four of them, made of neodymium and oriented so that the N and S poles alternate. Because this type of graphite is a diamagnetic material, an opposite magnetic field is produced and this causes a repulsion force that keep the feather light square suspended. The really clever part is the arrangement of the magnets which makes the arrangement self-stabilizing.
Source: http://www.scitoys.com
Size: 0.5"
Purity: 99.9%
|
|
 |
Diamond.
It came as a great surprise when the great French chemist Antoine Lavoisier demonstrated in a costly experiment in 1772 that when you burn diamonds you make carbon dioxide that is indistinguishable from the product of burning coal. The difference between coal and diamond is that in the latter, the carbon atoms form a 3D network of strong covalent bonds with one another to form a regular tetrahedral lattice. In coal and charcoal, by contrast, the bonding is more random and the resulting material has much less strength. The rough diamond here is a natural specimen that was formed a great temperatures and pressure deep underground.
Source: eBay
Size: 0.2"
Purity: >99%
|
|
 |
Coal.
This specimen of coal was kindly donated from stores at DePauw University.
Source: donated
Size: 1.5"
Purity: 85%
|
|

