|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Scandium is very, very expensive, quite a bit more than gold for example. This is somewhat odd since its abundance in the earth's crust is fairly high. The problem is that while there is a lot of it, it's spread out thinly, and there are very few places where it's concentrated enough to be extracted economically. It's also fairly difficult to isolate from its ores.
This is a pity, because if it were more common, it could be very useful. It's very strong, light, fairly stable in air, and would make a good substitute for things like aluminum, magnesium, and beryllium in aircraft and other applications where light weight are important. It's essentially non-toxic, unlike beryllium, and would probably make very fine tennis rackets, golf clubs, bicycle locks, and the like. If, that is, it didn't cost quite so much.
|
|
| |
|
|
|
|
|
Clipping of thin sheet.
Sometimes things just fall in your lap out of the clear blue sky. This morning I heard on the radio about a girl with an incredibly thick accent in England who had a small asteroid fall on her foot. This evening a friend I've know from highschool, Donald Barnhart, called up and asked if I'd like some scandium, because he had just picked up a clipping of it and thought I might like it. I said, um, yes, it was only the number one most wanted element on my list of most wanted elements!
The rest of the story about where it came from will have to remain untold, because the source wishes to remain anonymous.
On September 17, 2002 a fraction of this strip was de-accessioned from the Table to be sent to David Franco in small thanks for the many elements he has contributed.
Source: Anonymous
Contributor: Anonymous
Acquired: 29 August, 2002
Price: Donated
Size: 1"
Purity: 99.98%
|
|
|
|
|
|
|
Incredible crystals.
This is a truly lovely amount of scandium, ten grams, which would cost you over a thousand dollars from a chemical supplier. It was contributed by a party who wishes to remain anonymous. While it is very pure, 99.9%, that is not pure enough to be of any use in the industrial process their company is involved in, so they felt a contribution to the Periodic Table was in order. For which the Periodic Table is deeply appreciative: This is really an amazing hunk of a very rare substance. The crystals on it are quite remarkable.
The only thing I regret about it is that it's sealed under argon in a glass tube. The value and purity of the sample is such that I could never break the glass, which means I can never actually hold it in my hand. I'll just have to admire it from afar.
Here is a photograph taken through an inspection microscope of an even larger sample (not mine, unfortunately), worth tens of thousands of dollars. Confidentiality prevents me from describing anything more about the size or shape of the sample, only a portion of which you see here:
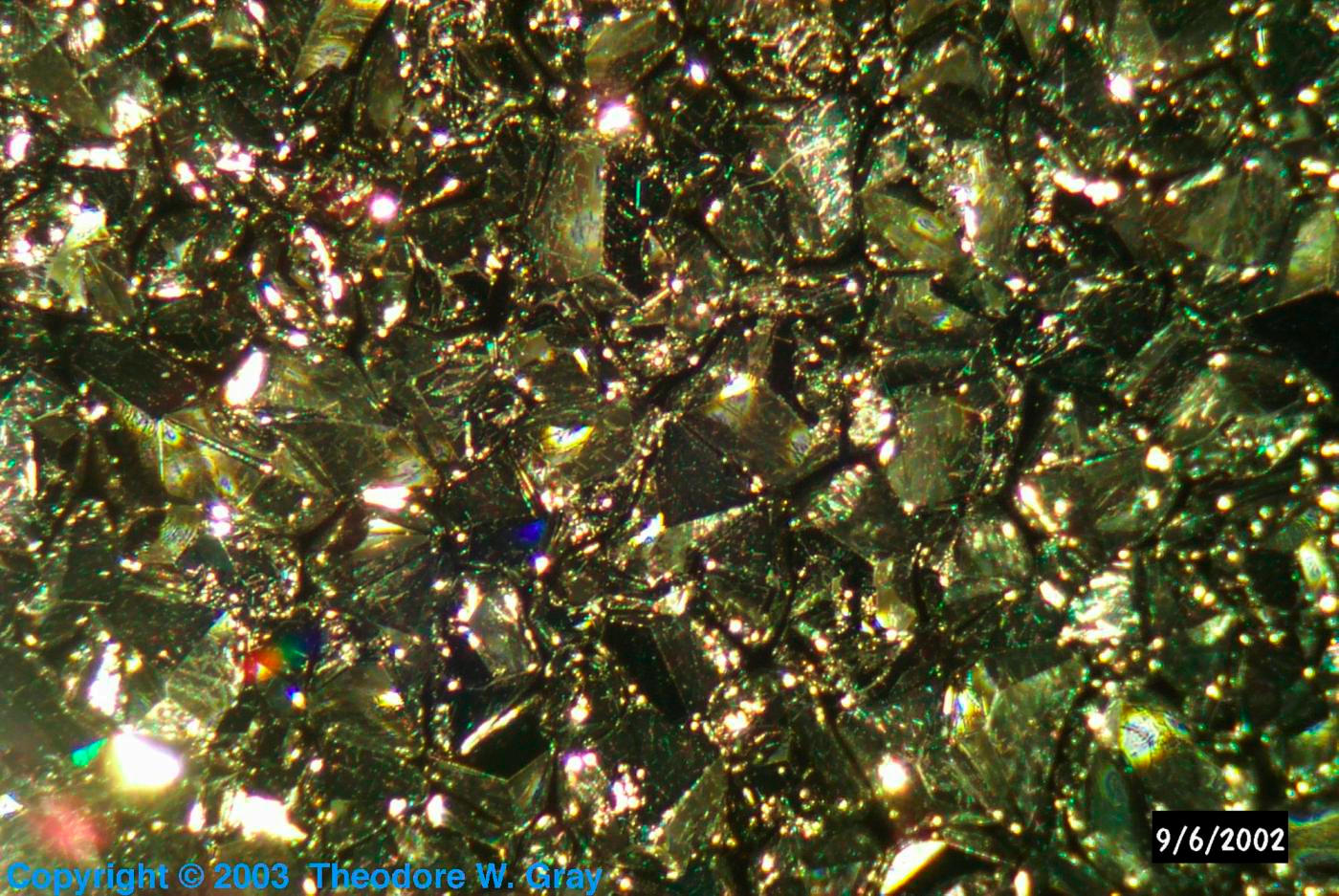
Source: Anonymous
Contributor: Anonymous
Acquired: 9 September, 2002
Price: Donated
Size: 1"
Purity: 99.9%
|
|
|
|
|
|
|
  Sample from the RGB Set. Sample from the RGB Set.
The Red Green and Blue company in England sells a very nice element collection in several versions. Max Whitby, the director of the company, very kindly donated a complete set to the periodic table table.
To learn more about the set you can visit my page about element collecting for a general description or the company's website which includes many photographs and pricing details. I have two photographs of each sample from the set: One taken by me and one from the company. You can see photographs of all the samples displayed in a periodic table format: my pictures or their pictures. Or you can see both side-by-side with bigger pictures in numerical order.
The picture on the left was taken by me. Here is the company's version (there is some variation between sets, so the pictures sometimes show different variations of the samples):

Source: Max Whitby of RGB
Contributor: Max Whitby of RGB
Acquired: 25 January, 2003
Text Updated: 11 August, 2007
Price: Donated
Size: 0.2"
Purity: 99.89%
|
|
|
|
|
|
|
Sample from the Everest Set.
Up until the early 1990's a company in Russia sold a periodic table collection with element samples. At some point their American distributor sold off the remaining stock to a man who is now selling them on eBay. The samples (except gases) weigh about 0.25 grams each, and the whole set comes in a very nice wooden box with a printed periodic table in the lid.
To learn more about the set you can visit my page about element collecting for a general description and information about how to buy one, or you can see photographs of all the samples from the set displayed on my website in a periodic table layout or with bigger pictures in numerical order.
Source: Rob Accurso
Contributor: Rob Accurso
Acquired: 7 February, 2003
Text Updated: 29 January, 2009
Price: Donated
Size: 0.2"
Purity: >99%
|
|
|
|
|
|
|
  Metal halide bulb. Metal halide bulb.
The most efficient currently available light sources are arc-discharge lights. The most efficient of all are low-pressure sodium vapor bulbs, but they give a very strongly yellow light that most people don't much like, perhaps because it makes everyone look dead. High pressure sodium lights and mercury vapor lights are better and only slightly less efficient.
The brilliant idea behind metal halide lights is to start with a mercury vapor light, which is very bright and very efficient, and mix in a pinch of this element and dab of that one, each with its own characteristic spectral lines, in order to build up out of individual emission lines a reasonably facsimile of the color of natural daylight. The result is a bulb that is only slightly less efficient than the most efficient low pressure sodium lights, but that gives a very pleasant natural daylight. And of course, the most important of those elements is scandium
Why don't you have any in your house? Generally they come only in very high wattages (400W, which gives an amount of light equivalent to a 1500W incandescent bulb, or higher). And worse, they take several minutes to warm up before you get much light at all. At the farm I have about ten 400W metal halide fixtures in the shop and four 1000W ones as yard lights: Producing that much high-quality light by any other technology would be impractical and hugely expensive in electricity, which is why this type of bulb is widely used in commercial and industrial settings (look up at most warehouse-style mega stores and you'll probably see an array of metal halide lamps).
There is one company, however, that sells a home-use, scandium-containing lamp: www.microsun.com, a division of the parent company of my favorite scandium supplier. I love the way their marketing department tries to make the slow-start sound like a feature: "Unlike other artificial lighting, the Microsun Metal Halide Light Source reaches full illumination gradually--resembling natural sunlight." Um, right. But aside from the slow start, metal halide light really is very attractive in many ways and it's ridiculously efficient, 4 or 5 times as efficient as incandescent light.
This 400W metal halide bulb is displayed in my Bulb Stand.
Source: Hardware Store
Contributor: Theodore Gray
Acquired: 28 November, 2003
Text Updated: 11 August, 2007
Price: $40
Size: 9"
Purity: <1%
Sample Group: Light Bulbs
|
|
|
|
|
|
|
Hollow cathode lamp.
Lamps like this are available for a very wide range of elements: Click the Sample Group link below to get a list of all the elements I have lamps like this for. They are used as light sources for atomic absorption spectrometers, which detect the presence of elements by seeing whether a sample absorbs the very specific wavelengths of light associated with the electronic transitions of the given element. The lamp uses an electric arc to stimulate the element it contains to emit its characteristic wavelengths of light: The same electronic transitions are responsible for emission and absorption, so the wavelengths are the same.
In theory, each different lamp should produce a different color of light characteristic of its element. Unfortunately, the lamps all use neon as a carrier gas: You generally have to have such a carrier gas present to maintain the electric arc. Neon emits a number of very strong orange-red lines that overwhelm the color of the specific element. In a spectrometer this is no problem because you just use a prism or diffraction grating to separate the light into a spectrum, then block out the neon lines. But it does mean that they all look pretty much the same color to the naked eye.
I've listed the price of all the lamps as $20, but that's really just a rough average: I paid varying amounts at various eBay auctions for these lamps, which list for a lot more from an instrument supplier.
(Truth in photography: These lamps all look alike. I have just duplicated a photo of one of them to use for all of them, because they really do look exactly the same regardless of what element is inside. The ones listed are all ones I actually have in the collection.)
Source: eBay seller heruur
Contributor: Theodore Gray
Acquired: 24 December, 2003
Price: $20
Size: 8"
Purity: 99.9%
Sample Group: Atomic Emission Lamps
|
|
|
|
|
|
|
 Aluminum-scandium master alloy. Aluminum-scandium master alloy.
This irregular lump comes from Tim Worstall, the world's leading expert on the scandium trade by virtue of the fact that he is a good fraction of the world's scandium traders. Virtually no pure scandium metal is sold in the world, most of the scandium that trades hands is either scandium oxide, or master alloys like this. A master alloy is a precisely formulated mixture of metals that is meant to be added to a pot of more common metal to form the desired final alloy. This one is aluminum with about 2% scandium added. Why not just buy pure scandium and add it to a pot of aluminum? After all, that would save a lot of weight shipping around 98 pounds of aluminum for every pound of scandium. But creating alloys from pure metals is not always so easy. For one thing, the melting point of scandium is much, much higher than that of aluminum: You'd have to heat your aluminum much higher than otherwise necessary in order to get it to absorb the scandium. By purchasing scandium pre-dissolved in aluminum, the end user can simply dump the master alloy chunks into their pot of aluminum near its normal melting point.
Aluminum-scandium alloys are used for things like very expensive bicycle frames, baseball bats, and even a line of light-weight handguns. (Amazingly, two separate readers have written in to me about this last application, both commenting that the guns are very uncomfortable to shoot because the light weight results in a strong kickback.) Tim is excited by the possibilities for expanded use of these alloys, including in things like aircraft wings.
Source: Tim Worstall
Contributor: Tim Worstall
Acquired: 19 January, 2004
Price: Donated
Size: 1.25"
Purity: 2%
|
|
|
|
|
|
|
|
|
|
  Element coin. Element coin.
Dave Hamric sells element samples under the name Metallium. He's developed a line of coins struck out of various common and uncommon metals: They are quite lovely, and very reasonably priced, considering the difficulty of creating some of them.
Here is the back side of this coin (click either picture to see it larger):

Click the Sample Group link below to see many other coins made of various elements, or click the link to his website above if you want to buy one like this.
Source: Dave Hamric
Contributor: Theodore Gray
Acquired: 24 December, 2007
Text Updated: 24 December, 2007
Price: $44
Size: 0.75"
Purity: >99%
Sample Group: Coins
|
|
|
|
|
|
|
|
|
|
  Microsun bulb. Microsun bulb.
The Microsun company sells table and floor lamps that use a small 68W metal halide lamp to create a lot of light, about as much as a 300W incandescent lamp would. These bulbs contain scandium made by friends of mine at a secretive company. The scandium contributes a large number of spectral emission lines to the light, giving it a pleasant sun-like appearance. The one problem is that metal halide bulbs take several minutes to come up to full brightness, not something the average person expects of their table lamp.
But it really is very nice light. I've gotten to the point that I can't stand dingy old yellow incandescent light anymore. Daylight spectrum compact fluorescent bulbs are nice, and these metal halide lights are as good or better than those. It will be interesting to see how good the spectral quality of the first practical home lighting LEDs is.
Source: Microsun
Contributor: Theodore Gray
Acquired: 8 February, 2009
Text Updated: 8 February, 2009
Price: $28
Size: 6"
Purity: <2%
|
|
|
|
|
|
|
  Dual-arc bulb. Dual-arc bulb.
This interesting bulb, meant for indoor plant growing operations, combines two different arc discharge tubes in one, to provide a wider spectrum of light than either type would alone. One is a high-pressure sodium arc, the other is a metal halide tube, probably containing scandium as a spectrum-enhancer.
Source: eBay seller garden_supply
Contributor: Theodore Gray
Acquired: 2 April, 2009
Text Updated: 3 April, 2009
Price: $94
Size: 12"
Purity: <2%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
  Insane mineral capsules. Insane mineral capsules.
These minerals capsules are called "Immune Boost 77", from Morningstar Minerals. They are either being incredibly honest, or they really don't understand what they're saying when they list what amounts to nearly the entire periodic table on the label, as the "trace minerals" they contain.
Here is the list in all its glory, typed in by my daughter in exchange for my paying for a membership in the Miley Cyrus fan club: Antimony, Barium, Beryllium, Bismuth, Boron, Bromine, Calcium, Carbon, Cerium, Cesium, Chloride, Chromium, Cobalt, Copper, Dysprosium, Erbium, Europium, Florine, Gadolinium, Gallium, Germanium, Gold, Hafnium, Holmium, Indium, Iodine, Iridium, Iron, Lanthanum, Lithium, Lutetium, Magnesium, Manganese, Molybdenum, Neodymium, Niacin, Nickel, Niobium, Osmium, Palladium, Phosphorus, Platinum, Potassium, Praseodymium, Rhenium, Rhodium, Rubidium, Ruthenium, Samarium, Scandium, Selenium, Silicon, Silver, Sodium, Strontium, Sulfur, Tantalum, Thallium, Thorium, Tellurium, Terbium, Thulium, Tin, Titanium, Tungsten, Vanadium, Ytterbium, Yttrium, Zinc, Zirconium.
Some of them are just silly, like thulium, which has absolutely no biological function. Others are a bit scarier, like thallium and thorium that are deadly poisons, and tellurium, which makes you smell of rotten onions for weeks.
Basically what they've done is list everything that occurs in even trace amounts in mixed monazite sand, which is kind of what the stuff inside looks like. The only reason they aren't seriously harmful (I assume) is that most of these are not actually present in any meaningful quantity.
My attention is drawn to these and other similar mineral supplements every time I decide to see if anything interesting has popped up on eBay for one or another of the obscure rare earths. Generally speaking if you search eBay for those guys you get very little of interest unless you turn on the option to search the text of the item description as well as the titles. Then you get lots of trace mineral supplements that one can only hope don't actually contain them.
Source: eBay seller grandma-adams
Contributor: Theodore Gray
Acquired: 24 March, 2009
Text Updated: 29 March, 2009
Price: $15
Size: 0.75"
Composition: SbCsDyErEuGdHfHoInLaLuNdPrSmScThTlTeTbTmYbY
|
|
|
|
|
|
|
|
|
|
  Himalayan sea salt. Himalayan sea salt.
There is a list of 84 elements that seems to pop up repeatedly in the ingredient lists of "natural" mineral products, supplements, pills, and the like. Even, it turns out, in salt. Here then is the list of minerals claimed to be found in all-natural organic Himalayan sea salt:hydrogen, lithium, beryllium, boron, carbon, nitrogen, oxygen, fluoride, sodium, magnesium, aluminum, silicon, phosphorus, sulfur, chloride, calcium, scandium, titanium, vanadium, chromium, manganese, iron, cobalt, nickel, copper, zinc, gallium, germanium, arsenic, selenium, bromine, rubidium, strontium, yttrium, zirconium, niobium, molybdenum, ruthenium, rhodium palladium, silver, cadmium, indium, tin, antimony, tellurium, iodine, cesium, barium, lanthanum, cerium, praseodymium, samarium, europium, gadolinium, terbium, dysprosium, holmium, erbium, thulium, ytterbium, lutetium, hafnium, tantalum, tungsten, rhenium, osmium, iridium, platinum, gold, mercury, thallium, lead, bismuth, polonium, astatine, francium, radium, actinium, thorium, protactinium, uranium, neptunium and plutonium. I wish someone would tell these people that, for example, neptunium and plutonium do not occur in nature at all, let alone in salt. Unless, I suppose, if you count nuclear fallout as a "natural" source of ingredients.
What bothers me most is what this says about the level of scientific literacy, both of the people selling the stuff, and the people buying it. Does no one actually read the list? Or do they read it an not realize how preposterous it is? It's enough to make you despair for the future of mankind.
Pretty salt, though.
Source: eBay seller saltwonders
Contributor: Theodore Gray
Acquired: 28 March, 2009
Text Updated: 4 April, 2009
Price: $15
Size: 0.25"
Composition: NaClSbCsDyErEuGdHfHoInLaLuNdPrSmScThTlTeTbTmYbY
|
|
|
|
|
|
|
|
|
|
|
|