|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
I have very fond memories of zinc because it's the first metal I melted and cast into many shapes as a wee lad. It's wonderfully suitable for casting because it's fairly non-toxic (certainly less toxic than lead), fairly low melting, and easy to get very cheap in the form of scrap roof flashing. Tin would be a better metal, but it's expensive and not available as scrap metal. (Click the first story book icon below for more about zinc casting.)
Aside from being an essential nutrient (first sign of zinc deficiency: your sense of smell goes), zinc is very widely used as an anti-corrosion plating for things make of iron. Contrary to popular belief, zinc plated objects are not intended or expected to last indefinitely. Zinc also oxidizes, just more slowly than iron, and the lifetime of a zinc plated ("galvanized") item is determined by how thick the zinc plating is.
Zinc is also used as a sacrificial anode to protect iron that can't be plated with it (for example, train tracks). See the oil tank anodes below for more on this.
|
|
| |
|
|
|
|
|
   Scrap roof flashing. Scrap roof flashing.
I've been melting and casting roof flashing zinc since the late 1970s. But I couldn't find any just now, so I purchased a new bucket of scrap at Marco's scrap metal in Champaign in April 2002. Later I found many pounds of it in my parents' basement. This metal was melted down and poured into mini-muffin tins to make nice little coins.
Click the book icon for a story about casting zinc and other metals, or find the mini-periodic table under zinc for a different story about casting.
Source: Marco's Scrap Metal
Contributor: Theodore Gray
Acquired: 15 April, 2002
Price: $1/pound for scrap zinc
Size: 1.25"
Purity: >90%
|
|
|
|
|
|
|
Post-1982 Pennies.
Before 1982, US pennies were made of solid copper. After the price of copper briefly went over a penny per penny, they reconsidered, and now pennies are copper plated zinc.
Source: America
Contributor: Theodore Gray
Acquired: 15 April, 2002
Text Updated: 18 December, 2007
Price: $0.01/penny
Size: 0.5"
Purity: >90%
Sample Group: Coins
|
|
|
|
|
|
|
  Art. Art.
I made this out of old roof flashing probably in the late 1970's. It's quite heavy, could definitely be used to defend yourself in a bar or something. You can actually see fingerprints that were in the wax I braided to cast it from, because I really didn't spend a lot of time on it: I just softened some wax, braided it up, and made a mold.
Source: Theodore Gray
Contributor: Theodore Gray
Acquired: 15 April, 2002
Price: $1/pound for scrap zinc
Size: 8"
Purity: >90%
|
|
|
|
|
|
|
  More Art. More Art.
Another thing I made out of old roof flashing probably in the late 1970's.
Source: Theodore Gray
Contributor: Theodore Gray
Acquired: 15 April, 2002
Price: $1/pound for scrap zinc
Size: 3"
Purity: >90%
|
|
|
|
|
|
|
Sample from the RGB Set.
The Red Green and Blue company in England sells a very nice element collection in several versions. Max Whitby, the director of the company, very kindly donated a complete set to the periodic table table.
To learn more about the set you can visit my page about element collecting for a general description or the company's website which includes many photographs and pricing details. I have two photographs of each sample from the set: One taken by me and one from the company. You can see photographs of all the samples displayed in a periodic table format: my pictures or their pictures. Or you can see both side-by-side with bigger pictures in numerical order.
The picture on the left was taken by me. Here is the company's version (there is some variation between sets, so the pictures sometimes show different variations of the samples):
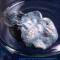
Source: Max Whitby of RGB
Contributor: Max Whitby of RGB
Acquired: 25 January, 2003
Text Updated: 11 August, 2007
Price: Donated
Size: 0.2"
Purity: 99.8%
|
|
|
|
|
|
|
Sample from the Everest Set.
Up until the early 1990's a company in Russia sold a periodic table collection with element samples. At some point their American distributor sold off the remaining stock to a man who is now selling them on eBay. The samples (except gases) weigh about 0.25 grams each, and the whole set comes in a very nice wooden box with a printed periodic table in the lid.
To learn more about the set you can visit my page about element collecting for a general description and information about how to buy one, or you can see photographs of all the samples from the set displayed on my website in a periodic table layout or with bigger pictures in numerical order.
Source: Rob Accurso
Contributor: Rob Accurso
Acquired: 7 February, 2003
Text Updated: 29 January, 2009
Price: Donated
Size: 0.2"
Purity: >99%
|
|
|
|
|
|
|
  Oil tank sacrificial anodes. Oil tank sacrificial anodes.
These little zinc balls are put into oil tanks to prevent rust: The idea is that the zinc oxidizes more easily than iron, so it gets consumed before the iron does. As long as you keep some zinc around, the iron will generally speaking not rust. The same idea is used to protect boat hulls, railroad tracks, and other steel structures. The zinc is simply buried in the ground near the iron and connected with a wire.
I chose this sample to represent its element in my Photographic Periodic Table Poster. The sample photograph includes text exactly as it appears in the poster, which you are encouraged to buy a copy of.

Source: eBay seller kaltofen
Contributor: Theodore Gray
Acquired: 14 February, 2003
Text Updated: 4 May, 2007
Price: $11/3
Size: 1"
Purity: >99%
|
|
|
|
|
|
|
|
|
|
  Mini periodic table table for Popular Science. Mini periodic table table for Popular Science.
In early 2003 I got an email from an editor at Popular Science column asking if I'd like to write a monthly column. Of course I said yes, and the second article (in the August, 2003 issue) is a story about lost wax casting as I did it when I was fifteen or so. For the photographs in the article I made a few little periodic table tables out of various metals: This one is made of zinc, and I also made ones out of copper, silver, and bismuth.
Source: Marco's Scrap Metal
Contributor: Theodore Gray
Acquired: 27 May, 2003
Price: $1/pound for scrap zinc
Size: 3"
Purity: >90%
|
|
|
|
|
|
|
Water dropped blobs.
I made these at a cookout at the farm to amuse some guests, by pouring just-molten zinc into a large stainless steel mop bucket (which I got at the closing out auction of the Lincoln Developmental Center in Lincoln Illinois, which was at one time the world's largest mental hospital, in case you're interested). The nodules are needle sharp! I poked myself on them as did the guests. When they first came out of the water they were bright and shiny, but unfortunately being zinc they did not stay that way: This picture was taken just a couple of days later and as you can see, they are already starting to turn dull and gray.
The shapes you get from water-dropped metals are quite variable, from rounded aluminum blobs to stringy lead ropes. It also depends on how high above the melting point the metal is: For example if you heat aluminum to well beyond its melting point before pouring into water, it fragments into almost a powder when it hits the water, while if it's just barely molten, you get smooth round nodules. This zinc was just barely above its melting point.
Source: Marco's Scrap Metal
Contributor: Theodore Gray
Acquired: 20 July, 2003
Price: $1/pound for scrap zinc
Size: 0.75"
Purity: >90%
|
|
|
|
|
|
|
Anode balls.
These are zinc anode balls of very high purity: 99.99% guaranteed. It's a far cry from the scrap roof flashing for which I still hold font memories. But let's face it, at less than a dollar a pound in bulk from a wholesale distributor, this stuff is much, much more pleasant to work with. It think it even tarnishes more slowly than impure zinc from the scrap yard. See below for one of the first things I made with this new source of high-purity zinc.
Source: sulfuric.com
Contributor: Theodore Gray
Acquired: 9 August, 2003
Price: $1/pound
Size: 2"
Purity: 99.99%
|
|
|
|
|
|
|
Link in multi-metal chain.
I had been wondering about how hard it would be to make a multi-part graphite mold with which I could cast chain links around each other. That is, given an existing link, cast a new one interlinked with it. This turns out to be quite do-able: Here is the mold I made (using my drill press as a vertical mill and a round-ended router bit):
 
In case you ever want to try this, I'll give you an important hint: The third link is the real test, not the second one.
Using this mold I have cast a chain out of all the metals I can easily cast. Click the Sample Group link below to see all the links together.
This chain (counted as one sample) is the 600th sample added to my collection.
Source: sulfuric.com
Contributor: Theodore Gray
Acquired: 9 August, 2003
Text Updated: 20 February, 2006
Price: $1/pound
Size: 3"
Purity: 99.99%
Sample Group: Multi-metal Chain
|
|
|
|
|
|
|
Outboard motor anode.
This is another example of a sacrificial anode, which electrochemically protect a more valuable (typically steel) object from corrosion. In this case, the object to be protected is an outboard motor. How often the anode has to be replaced depends on the kind of water the motor is used in: Around here I am told they last pretty much forever.
Source: Boat Shop
Contributor: Theodore Gray
Acquired: 22 August, 2003
Price: $10
Size: 2"
Purity: 99%
|
|
|
|
|
|
|
Larger boat anode.
Yet another sacrificial anode, this one from navy surplus and thus probably intended for large ships. It weighs about 4.5 pounds.
Source: eBay seller artone03
Contributor: Theodore Gray
Acquired: 17 November, 2003
Price: $5
Size: 5"
Purity: 99%
|
|
|
|
|
|
|
Even larger boat anode.
This sacrificial anode is getting into the "really large" size range at 13 pounds. It is helpfully stamped "do not paint" because of course if you did paint it, it would no longer be in electrical contact with the water around it, and therefore it could not perform its duty, which is to be corroded and eaten away instead of the iron ships hull it is protecting.
Source: eBay seller precision-marine
Contributor: Theodore Gray
Acquired: 17 November, 2003
Price: $5
Size: 5"
Purity: 99%
|
|
|
|
|
|
|
Zinc-air hearing aid batteries.
Batteries generate electricity by allowing two chemicals to react with each other in a way that releases electrons, which are tapped and fed through a wire to do useful work. In most batteries both chemicals are present in the battery and they stay there: It's a sealed system. Fuel cells, like the hydrogen-oxygen fuel cells that are looking so promising, use the same idea except instead of being sealed, they accept a continuous flow of the two chemicals from storage tanks: This allows them to operate indefinitely.
These zinc-air batteries are exactly half way in between. The chemical reaction they use is the oxidation of zinc metal by the oxygen in air. The zinc is built in to the battery and can't be replaced, while the air flows in as needed to replenish air used up in generating electricity. So, it's a fuel cell with regards to oxygen and a conventional battery with regards to zinc.
These batteries provide a very large amount of energy per unit weight, because they only have to carry half the chemicals, the rest coming from the air. It's the same reason jet engines can go further than rockets on the same weight of fuel: The jet is taking half its "fuel" from the surrounding air. And just like with jets and rockets, the reason not all batteries are zinc air batteries is that you can't get as much instantaneous power out of them, because you have to wait for enough air to get into the system.
In a conventional battery you can draw a very large amount of current if you want, because both chemicals you need are right there ready to react. Same in a rocket. In a zinc-air battery you can only draw as much current as their is air flow to sustain it. Same in a jet engine. As a result, zinc-air batteries are used in applications that require slow, steady amounts of power, like a hearing aid or monitoring device. Similarly, jet engines are used in places where you need a steady amount of power for a long time, like a transatlantic flight. Rockets are used when you want a huge amount of power in a very short time, or, of course, in places where there is no air. That's right, zinc-air batteries would not work in space.
Source: eBay seller free-shipping-auctions
Contributor: Theodore Gray
Acquired: 15 January, 2004
Text Updated: 11 March, 2007
Price: $10/32
Size: 0.5"
Purity: 10%
Sample Group: Medical
|
|
|
|
|
|
|
8.4V zinc-air batteries.
These batteries are the size and shape of a standard 9V battery, but they are air-breathing half-fuel cells instead. See the previous sample for more information about how zinc-air batteries work. Note that these come sealed in a metalized Mylar vacuum pack: As long as the package remains unopened their shelf-life is virtually unlimited, because one of the components needed to make the battery work, air, has been excluded. My understanding is that after you open the package it takes a couple of minutes for the voltage to build up, then the battery is ready to use.
Source: eBay seller dmhsgeneralstore
Contributor: Theodore Gray
Acquired: 15 January, 2004
Text Updated: 29 January, 2009
Price: $6/3
Size: 2"
Purity: 10%
|
|
|
|
|
|
|
 Museum-grade sample. Museum-grade sample.
In early 2004 Max Whitby and I started selling individual element samples identical or similar to the samples we use in the museum displays we build. These are top-quality samples presented in attractive forms appropriate to the particular element. They are for sale from Max's website and also on eBay where you will find an ever-changing selection of samples (click the link to see the current listings).
This ingot shows two kinds of surfaces, one where the metal cooled in contact with a graphite mold, and the top that was in contact with the air. The top surface is particularly interesting in that it shows the degree of contraction when the metal cools and sometimes crystal structures that give the surface a wrinkled appearance as in copper or tin.
Source: Theodore Gray
Contributor: Theodore Gray
Acquired: 6 March, 2004
Text Updated: 11 August, 2007
Price: See Listing
Size: 4"
Purity: >99%
Sample Group: RGB Samples
|
|
|
|
|
|
|
 Museum-grade sample. Museum-grade sample.
In early 2004 Max Whitby and I started selling individual element samples identical or similar to the samples we use in the museum displays we build. These are top-quality samples presented in attractive forms appropriate to the particular element. They are for sale from Max's website and also on eBay where you will find an ever-changing selection of samples (click the link to see the current listings).
This ingot is designed to show what the surface of the metal looks like when hammered: By comparing with similar hammered ingots of other metals an idea of the hardness and working characteristics of the metal is given. (We use approximately the same hammering force on each ingot, so lead will show much deeper hammer marks than zinc, for example.)
Source: Theodore Gray
Contributor: Theodore Gray
Acquired: 6 March, 2004
Text Updated: 11 August, 2007
Price: See Listing
Size: 4"
Purity: >99%
Sample Group: RGB Samples
|
|
|
|
|
|
|
 Museum-grade sample. Museum-grade sample.
In early 2004 Max Whitby and I started selling individual element samples identical or similar to the samples we use in the museum displays we build. These are top-quality samples presented in attractive forms appropriate to the particular element. They are for sale from Max's website and also on eBay where you will find an ever-changing selection of samples (click the link to see the current listings).
This "crystal nest" is formed by allowing a cup of the molten metal to half-solidify, then pouring the liquid center off. Different metals form surprisingly diverse types of crystals using this very simple technique. The size and shape of the crystals is very sensitive to the purity of the material: This bullet lead is relatively low purity and therefor forms relatively small crystals: As a rule the higher the purity, the larger the crystals, with the most spectacular example being bismuth of 99.99% or better purity.
Source: Theodore Gray
Contributor: Theodore Gray
Acquired: 6 March, 2004
Text Updated: 11 August, 2007
Price: See Listing
Size: 3"
Purity: >99%
Sample Group: RGB Samples
|
|
|
|
|
|
|
 Museum-grade sample. Museum-grade sample.
In early 2004 Max Whitby and I started selling individual element samples identical or similar to the samples we use in the museum displays we build. These are top-quality samples presented in attractive forms appropriate to the particular element. They are for sale from Max's website and also on eBay where you will find an ever-changing selection of samples (click the link to see the current listings).
This cast ball is designed to show what the surface of the metal looks like when hammered: By comparing with similar hammered balls of other metals an idea of the hardness and working characteristics of the metal is given. (We use approximately the same hammering force on each ball, so lead will show much deeper hammer marks than zinc, for example.)
Source: Theodore Gray
Contributor: Theodore Gray
Acquired: 1 May, 2004
Text Updated: 11 August, 2007
Price: See Listing
Size: 2"
Purity: >99%
Sample Group: RGB Samples
|
|
|
|
|
|
|
Mini element collection.
This is a nice little set from the 1960's. The enclosed price list indicates it cost a few dollars, and the enclosed mercury sample indicates it predates current environmental concerns! Here's a picture of the whole 2-box set:

Source: Blake Ferris
Contributor: Theodore Gray
Acquired: 15 July, 2004
Price: $61/set
Size: 1"
Purity: >98%
|
|
|
|
|
|
|
Another anode.
This is an unusually attractive zinc anode, meant to be bolted to a ship's hull to prevent oxidation (rusting) of the hull.
Source: eBay seller artone03
Contributor: Theodore Gray
Acquired: 10 August, 2004
Price: $7
Size: 8"
Purity: >98%
|
|
|
|
|
|
|
Assorted glow-in-the-dark paints.
This lovely array of glow-in-the-dark (phosphorescent) powders illustrates the range of colors and the brightness of modern luminous paints. Green and aqua are europium doped strontium aluminate, the brightest of all the modern phosphorescent powders. Blue is a alkali earth silicate, while red and orange are older, noticeably less bright zinc sulfides. (The difference in brightness is so great it was difficult to get a photograph that showed the glowing of the zinc sulfide without overexposing the other colors!) The powder packets are meant to be mixed with paint, nail polish, or whatever, rendering them luminous. The bottle in the back is ready-made paint, while the small tub is a heat-and-dip powder.
This set was kindly donated by Ready Set Glo: Visit their website at www.readysetglo.com or their eBay store.
(The "purity" listed below doesn't mean a whole lot since this is a mixture of several different compounds: I'm just indicating that this sample is not a simple element but rather a mixed compound.)
Source: Ready Set Glo
Contributor: Ready Set Glo
Acquired: 15 August, 2004
Text Updated: 11 August, 2007
Price: Donated
Size: 4"
Purity: <50%
|
|
|
|
|
|
|
Pretty little ingot.
This is a small bar of zinc that was clearly meant to be some kind of commemorative or advertising item, presumably for the Bunker Hill company/mine/town/whatever. The surface has been etched to bring out the crystal patterns, and it has little felt feet on it. Obviously a bar meant to be appreciated, not melted down. It's fairly high purity for zinc, if we believe the 99.99 molded in it.
Source: eBay seller markbritt
Contributor: Theodore Gray
Acquired: 16 August, 2004
Price: $6.50
Size: 4"
Purity: 99.99%
|
|
|
|
|
|
|
  Little animals. Little animals.
For a filming project with Max Whitby I made a bunch of small zinc animals using a cornbread mold. The raw material was the 2" anode balls (see above), of which I have a large box. The mold is made of cast iron with a Teflon non-stick coating (unaffected by molten zinc) from Williams-Sonoma.
Source: Theodore Gray
Contributor: Theodore Gray
Acquired: 15 September, 2005
Text Updated: 29 January, 2009
Price: Donated
Size: 3"
Purity: 99.95%
|
|
|
|
|
|
|
Concrete parting tool.
This is a tool used to create the sort of line you see in sidewalks about every 4-5 feet. After the concrete is partly set, but still soft, you run this tool along a straight board held over the concrete, forming a neat line with rounded edges. The purpose of this line is to weaken the concrete along that line, so that when the concrete cracks, it will do so at the bottom of the scribed line, where you won't see the crack. (With concrete slabs larger than a certain size, it's not a question of if, but when, it will crack. Better to have neat-looking, regularly spaced lines that look like you meant for them to be there, than irregularly spaced cracks that look like obvious defects. It's purely a question of hiding flaws, nothing that actually improves the performance of the concrete.)
This tool says on its label that it's made of zinc, and the surface looks a lot like zinc to me. It's quite heavy, presumably to make it easier to push along the concrete without riding up. Alloys referred to as "pot metal", which often contain zinc, are commonly used to cast simple shapes like this which don't need to be particularly strong, but this one claims to be all zinc, which is unusual and makes me think maybe it is. No idea why this would be better than pot metal: Maybe it's cheaper.
Source: Hardware Store
Contributor: Theodore Gray
Acquired: 28 October, 2005
Price: $20
Size: 6"
Purity: 95%
|
|
|
|
|
|
|
  Crow's foot zinc. Crow's foot zinc.
The February 2007 issue of Popular Science magazine (for which I write a monthly column has my article about making an antique-style "gravity cell", a type of battery made with a solution of copper sulfate and an electrode made of cast zinc. These were known a hundred years ago as "crow's foot zincs", and were available at that time in any corner general store. But these batteries have not been used for at least 60 years, and not even on eBay can you buy a crow's foot zinc, so I was forced to machine a graphite mold to use to cast my own brand new copies, based on a photograph I found on the internet. (Within days of the article reaching subscribers I got an email from someone wondering where I got the crow's foot zincs, and if they could get some too. I had to tell them that I've probably made the first new ones manufactured in many decades, and so far as I know there is simply no source for them. Except, of course, my little graphite mold.)
Actually there's no need for such a fancy shape of electrode, any old hunk of zinc would work reasonably well. I was just showing off by making a replica of an antique style electrode: I wanted the pictures to look pretty in the magazine.
Source: Theodore Gray
Contributor: Theodore Gray
Acquired: 1 December, 2006
Text Updated: 14 January, 2007
Price: $0.10
Size: 3"
Purity: 99.99%
|
|
|
|
|
|
|
  Element coin. Element coin.
Dave Hamric sells element samples under the name Metallium. He's developed a line of coins struck out of various common and uncommon metals: They are quite lovely, and very reasonably priced, considering the difficulty of creating some of them.
Here is the back side of this coin (click either picture to see it larger):

Click the Sample Group link below to see many other coins made of various elements, or click the link to his website above if you want to buy one like this.
Source: Dave Hamric
Contributor: Theodore Gray
Acquired: 1 December, 2006
Text Updated: 14 January, 2007
Price: $9
Size: 0.75"
Purity: >99%
Sample Group: Coins
|
|
|
|
|
|
|
  Zinc penny, modern. Zinc penny, modern.
This is an amazing object: A modern (1990) penny from which the copper plating has been completely removed. US pennies made after 1982 have a zinc core with a copper plating over it. Removing the zinc from inside, leaving a thin foil of copper, is actually quite easy: Just sand off some of the copper around the edge and then soak the penny in hydrochloric acid (HCl, also called muriatic acid in hardware stores). I have an example of that listed under copper.
But doing the converse is much harder. Any acid that will dissolve copper will dissolve zinc much faster, so it cannot be done using acid. I was at a loss, and the internet did not reveal anyone else who had done it. Fortunately Tryggvi, my chemist friend, took on the project, and found a way using cyanide complexation. Cyanide ions wrap themselves tightly around copper ions in solution, forming what's known as a complex. They do not, on the other hand, care much for zinc at all. In a concentrated solution of potassium cyanide copper is rendered slightly more vulnerable to oxidation than zinc. Under just the right conditions (cool temperatures and gentle agitation) the oxidizer potassium persulfate, combined with concentrated potassium cyanide, will slowly remove copper without touching zinc. It took Tryggvi many tried, and many ruined pennies, to get the procedure just right, but the result was perfection itself.
See the next sample for a clever example of what you can do with disassembled pennies.
Source: Tryggvi Emilsson and Timothy Brumleve
Contributor: Tryggvi Emilsson and Timothy Brumleve
Acquired: 4 May, 2007
Text Updated: 9 May, 2007
Price: $0.01
Size: 0.6"
Purity: 99%
Sample Group: Coins
|
|
|
|
|
|
|
  Split penny. Split penny.
There's a guy, Gunther von Hagens, who creates museum exhibits that consist of human bodies that have been split open and displayed as perfectly preserved cross sections or expanded versions of their natural state. This is the same idea, only applied to a 1990 US penny.
Modern pennies consist of a zinc core with a copper plating. See the previous sample for information about how remove the copper and leave the zinc core, and this sample for information about how to remove the zinc core and leave the copper foil.
Do both and glue them together with a tiny stick of pencil lead, and you have this expanded penny, which I used in a Popular Science column. Watch the rotation video to see if from all sides.
Source: Tryggvi Emilsson and Timothy Brumleve
Contributor: Tryggvi Emilsson and Timothy Brumleve
Acquired: 4 May, 2007
Text Updated: 11 August, 2007
Price: $0.03
Size: 0.6"
Purity: 99%
Sample Group: Coins
|
|
|
|
|
|
|
  Chrome plated zinc ashtray. Chrome plated zinc ashtray.
This lovely chrome plated ashtray is probably made of an alloy that is primarily zinc, but I'm only guessing at the percentage. The lettering around the center says St. Louis Die Casting Corp, Aluminum Zinc Magnesium. It's too heavy to be aluminum or magnesium, which leaves zinc, or one of the inexpensive pot metal alloys that are based on it.
Source: James Weigner
Contributor: James Weigner
Acquired: 9 May, 2007
Text Updated: 9 May, 2007
Price: Donated
Size: 8"
Purity: >90%
|
|
|
|
|
|
|
|
|
|
  Candle wicks with zinc cores. Candle wicks with zinc cores.
Apparently candle wicks sometimes need to be made stiffer than they ordinarily would be, and thus have wire cores put in them. I first heard about this in connection with a recall of candles that turned out to contain lead wires, which turn into lead vapor as the candle is burned. This used to be common, but is now considered something to panic about, due to the toxic nature of lead.
This candle wick, one of a pack of 100 I got on eBay, supposedly has a zinc wire, not lead, which will burn up into relatively harmless zinc oxide as the candle burns. (In high concentrations over long periods of time inhaled zinc oxide can be harmful, but it's not a cumulative poison in the way lead is, so very low levels of exposure such as you would get from a candle do not add up to a problem over the long run.)
Source: eBay seller angellitcandles
Contributor: Theodore Gray
Acquired: 23 December, 2007
Text Updated: 23 December, 2007
Price: $9.25/100
Size: 3"
Purity: >90%
|
|
|
|
|
|
|
  Bridgeman-process Zinc Crystal. Bridgeman-process Zinc Crystal.
I'm not sure what the Bridgeman process is, but that is said to be how this bar of crystalline zinc was produced.
Source: Anonymous
Contributor: Theodore Gray
Acquired: 8 March, 2008
Text Updated: 8 March, 2008
Price: Anonymous
Size: 1.5"
Purity: >99.9%
|
|
|
|
|
|
|
|
|
|
  Zinc medallion. Zinc medallion.
For a Popular Science column I wrote about making glass I needed a graphite mold to press the glass into. I machined one out of a block of graphite, and tested it by casting this medallion out of cheap and easy to melt zinc. Satisfied with the shape I went on to make similar medallions out of glass (listed under silicon because glass is mostly silica).
Source: Theodore Gray
Contributor: Theodore Gray
Acquired: 26 October, 2008
Text Updated: 29 January, 2009
Price: $1
Size: 2.5"
Purity: 99.9%
|
|
|
|
|
|
|
  Model Liberty Bell. Model Liberty Bell.
Why is a model of the bronze Liberty Bell listed under zinc? Because this is a cheap trinket probably cast in zinc-based pot metal.
Source: Philadelphia Airport
Contributor: Theodore Gray
Acquired: 28 February, 2009
Text Updated: 1 March, 2009
Price: $4
Size: 3"
Purity: 80%
|
|
|
|
|
|
|
|
|
|
  Electroluminescent fractal sculpture. Electroluminescent fractal sculpture.
This beautiful 3D Hilbert fractal in electroluminescent plastic was a gift from Richard Crandall, a long-time Mathematica user and Apple fellow who also has a business, Perfectly Scientific, which sells algorithms, lab equipment, and scientific art, including this lovely object.
Click the Source link to see two other variations of the 3D Hilbert space filling fractal.
Source: Perfectly Scientific
Contributor: Ethan Currens
Acquired: 24 March, 2009
Text Updated: 24 March, 2009
Price: Donated
Size: 4"
Purity: <20%
|
|
|
|
|
|
|
|
|
|
  Ordinary zinc-plated bolt and nut. Ordinary zinc-plated bolt and nut.
This is a perfectly ordinary hardware-store variety galvanized (zinc-plated) steel bolt and nut.
Source: Farm & Fleet
Contributor: Theodore Gray
Acquired: 8 April, 2009
Text Updated: 9 April, 2009
Price: $1
Size: 2"
Purity: <1%
|
|
|
|
|
|
|
  Cast candlestick. Cast candlestick.
This is a small zinc candlestick made by lost wax casting. Chris Anderson and I made several small zinc castings way, way back when, and this is one that he made and kept for decades before deciding that it belonged photographed and stored in my collection.
Source: Chris Anderson
Contributor: Chris Anderson
Acquired: 13 January, 2010
Text Updated: 13 January, 2010
Price: Donated
Size: 3"
Purity: >95%
|
|
|
|
|
|
|
|
|
|
|
|
|
63 pound solid brass cylinder.
The first question is, "is it hollow?", followed rapidly by "is it glued down?". No, it's solid and no, it's just really heavy. I got this as a dirty, rough cutoff at Marco's scrap metal, but it cleaned up pretty nicely with a combination of power and hand sanding to 600 grit, followed by buffing with some polishing compounds.
The density of brass is really not that much less than lead, which makes this cylinder a nice complement to my lead doorstop.
Source: Marco's Scrap Metal
Contributor: Theodore Gray
Acquired: 21 August, 2003
Price: $35
Size: 10"
Composition: CuZn
|
|
|
|
|
|
|
  Brass shell casings. Brass shell casings.
This is a collection of assorted shell casings made of brass (a copper-zinc alloy). In use, casings like this would be filled with gunpowder or other propellent, with a projectile (bullet) fastened in the open end.
Source: Derek McKinley
Contributor: Derek McKinley
Acquired: 13 February, 2007
Text Updated: 14 February, 2007
Price: Donated
Size: 2"
Composition: CuZn
|
|
|
|
|
|
|
  Brass split ring. Brass split ring.
The Boeing aircraft company operates a wonderful surplus store in Kent, Washington (near Seattle). I have no idea why they made, and then had second thoughts about using, this lovely huge, heavy, split ring of brass, but they did, and it ended up in their surplus store. It looks like something you could make a magnet out of, if it were iron instead of brass.
Source: Boeing Surplus Store
Contributor: Theodore Gray
Acquired: 11 August, 2007
Text Updated: 11 August, 2007
Price: $15
Size: 7"
Composition: CuZn
|
|
|
|
|
|
|
|
|
|
|
|
|
  Hemimorphite. Hemimorphite.
Description from the source:
Hemimorphite (Zn4 Si2 O7 (OH)2 x H2 O orth.), Ojuela Mine, Mapimi`, Durango, Mexico. Transparent, perfect crystals on limonitic matrix. 4,5x3x2 cm; 22 g.
Source: Simone Citon
Contributor: John Gray
Acquired: 30 September, 2008
Text Updated: 1 October, 2008
Price: Trade
Size: 1.75"
Composition: Zn4Si2O7(OH)2.H2O
|
|
|
|
|
|
|
  Smithsonite. Smithsonite.
Description from the source:
Smithsonite (Zn CO3 trig.), Monroe Co., USA. Botryoidal, translucent crystals on matrix. 1,5x1,5x1 cm; 9 g with box.
Source: Simone Citon
Contributor: John Gray
Acquired: 26 October, 2008
Text Updated: 26 October, 2008
Price: Trade
Size: 0.6"
Composition: ZnCO3
|
|
|
|
|
|
|
|
|
|
|
|
|
  Renierite. Renierite.
Description from the source:
Renierite ((Cu,Zn)11(Ge,As)2Fe4S16 tetr.), Kipushi, Shaba, Dem. Rep. of Congo. Perfect example, with brown-orange-reddish cristalline masses. 1,5x1,2x1 cm; 3 g.
Source: Simone Citon
Contributor: John Gray
Acquired: 28 January, 2009
Text Updated: 29 January, 2009
Price: Trade
Size: 0.6"
Composition: (Cu,Zn)11(Ge,As)2Fe4S16
|
|
|
|
|
|
|
  Smithsonite. Smithsonite.
Description from the source:
Smithsonite (Zn CO3 trig.), Laurion, Grecia. Light green botryoidal, from old Cu Pb Zn deposit. 6,5x6x2 cm; 48 g.
Source: Simone Citon
Contributor: John Gray
Acquired: 28 January, 2009
Text Updated: 29 January, 2009
Price: Trade
Size: 2.6"
Composition: ZnCO3
|
|
|
|
|
|
|
  Density Set. Density Set.
A cute little set of six cubes made from different metals, used to show students how different their densities can be. For cost reasons these sets rarely contain any really dense elements, such as tungsten, which is a pitty since students thus come away with the idea that lead is the densest metal, which is far from the truth. Osmium is twice as dense, and tungsten a good 75% more dense.
Source: Educational Innovations
Contributor: Theodore Gray
Acquired: 11 March, 2009
Text Updated: 12 March, 2009
Price: $20
Size: 1"
Composition: PbCuFeZnAlZn
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
  Davy Lamp. Davy Lamp.
An antique "Davy lamp", named after Sir Humphry Davy and used by miners before the invention of electric lights. The wire mesh, against all odds, prevents the flame from igniting flammable gases surrounding the lamp in a mine.
Source: eBay seller curiodream100
Contributor: Theodore Gray
Acquired: 13 January, 2010
Text Updated: 13 January, 2010
Price: $50
Size: 10"
Composition: CuZnCH
|
|
|
|
|
|
|
|
|
|
|
|