|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Copper is a wonderful metal. As one of the very few elements that are colored in their pure form, copper has a natural edge over other metals in the attractiveness department. It's also stable in air and water (though it does tarnish some, it won't rust away the way iron does), highly malleable (which means you can bend and shape it easily), easy to cast, and reasonably inexpensive.
The definitive use of copper is of course for electrical wiring. Silver is actually a better electrical conductor than copper, but of course it's far more expensive and isn't used for wiring. Aluminum isn't as good a conductor as copper, but it's a lot cheaper and lighter, and for that reason aluminum is quite commonly used for wiring, especially for the thick wires used for the main service entrance for residential and commercial electrical supplies.
For a brief period of time aluminum wire was promoted for use inside houses for hooking up individual circuits: This turned out to be a disaster because it turned out that copper's malleability and oxidation resistance are just as important as its conductivity for electrical safety. The aluminum wires became fragile where they were bent to make connections, and they formed insulating oxide layers: Both points had increased electrical resistance which had the potential to overheat and start house fires.
So house wiring is back to all copper, and only copper.
|
|
| |
|
|
|
|
|
  3/0 electrical wire. 3/0 electrical wire.
This wire was left over from when I rewired the main service entrance at the farm, and installed the backup generator in the early 1990s.
It's been melted down and left to cool in the standard graphite crucible used for several metal samples. The cylinders came out very clean and required only a power wire brushing to bring out the shine. Coated with a light coat of oil to retard tarnishing.
Copper is a good metal for casting: See zinc for more about metal casting.
Source: Hardware Store
Contributor: Theodore Gray
Acquired: 15 April, 2002
Price: $1.50/foot
Size: 1.25"
Purity: >99%
|
|
|
|
|
|
|
 Pre-1982 Pennies. Pre-1982 Pennies.
Before 1982, US pennies were made of solid copper. After the price of copper briefly went over a penny per penny, they reconsidered, and now pennies are copper plated zinc.
Source: America
Contributor: Theodore Gray
Acquired: 15 April, 2002
Text Updated: 18 December, 2007
Price: $0.01/penny
Size: 0.5"
Purity: >90%
Sample Group: Coins
|
|
|
|
|
|
|
Native Copper.
Found on a beach on one of the Apostle Islands in Lake Superior.
Source: Chris Carlson
Contributor: Lena Carlson
Acquired: 11 July, 2002
Price: Donated
Size: 0.6"
Purity: >90%
|
|
|
|
|
|
|
|
|
|
 Michigan Native Copper. Michigan Native Copper.
A Christmas present element: This ball came with the following tag:Michigan's Keweenaw Peninsula is the most important locality in the world for pure native copper. Mining began in 1845 and continued until 1968 and over 5 million tons of refined copper were recovered from Michigan's copper mines. In the early 1900's, it led the world in copper production and "Lake Copper" was the purity standard to which other refined copper was compared.
Long before the mining operations of the past century began in the Keweenaw, prehistoric peoples mined copper from the mineral-rich veins. It looks to me like this ball was melted down, probably from many small bits of native copper. Larger nuggets are much more valuable in their natural state, and are usually cut and polished, then sold for hundreds of dollars.
Source: John Gray
Contributor: John Gray
Acquired: 25 December, 2002
Text Updated: 29 January, 2009
Price: Donated
Size: 1"
Purity: >90%
|
|
|
|
|
|
|
  Arizona Raspberry Copper Nodules/Nuggets. Arizona Raspberry Copper Nodules/Nuggets.
Really strange looking nodules. The distinction between nodules and nuggets is that nodules are formed by precipitation or other chemical action while nuggets are formed by mechanical action in the solid state. At least, I think that's the distinction implied by this letter from the US Geological Survey, which offered an opinion on these things back in 1978 (click on the picture to get a readable version in a separate window):

A possible history of these rather strange nuggets is told by this letter, supplied by the eBay seller (who has more if you email him at coppernuggets@yahoo.com):

The seller supplied a copy of an analysis indicating them to be 94.8% copper, but not saying what the rest was, other than that it wasn't palladium, platinum, or rhodium. I confirmed the analysis by x-ray fluorescence spectroscopy at the Center for Microanalysis of Materials, University of Illinois (partially supported by the U.S. Department of Energy under grant DEFG02-91-ER45439), getting a result of 94.9% copper, 5.1% iron. The iron explains the fact that they are slightly magnetic.
And they certainly are quite curious and pretty, whether the story is 100% true or not, which as you will see is not particularly likely.
I cut one in half (actually I ground half of it away with a bench grinder), and this is what it looks like inside:

According to the USGS letter, the core is some mixture of copper oxides, but clearly the bulk of it is metallic copper. I made a QuickTime VR 3D image of the sliced version (warning, it's a high-resolution, 12MB file).
By way of fact-checking, I looked up the name on the USGS letter, and (probably on account of the unusual name), got lucky with the first email address I tried. The response was great:Yes, I am the same person although doing very different things now . I have my own business (www.specmin.com) and no longer work for the USGS.
I definitely do remember those nuggets.
We never saw anything like them again, to the best of my recollection.
In subsequent years, I have become a consultant to the mining industry and traveled extensively in South America to different mines and have not seen anything like them there either.
I have sent this out to a few of my copper industry friends and other contacts to see if they have
I will let you know if I get a response
Phoebe Hauff She's going to get some of the nuggets and show them around to her mining industry friends and of course I'll report anything that comes up.
Coincidentally I later got an email on an unrelated subject from another person, Joe Taggart, who currently works at the USGS. It turns out he knew Phoebe when she worked there. He didn't see the nodules when they had them in 1978, but based on the pictures on my website and Phoebe's letter, he said they looked like they might be pseudomorphs, clusters of cuprite crystals which were then converted into copper metal by the action of a reducing solution.
At that point I thought I knew the answer for sure: They were naturally occurring copper nodules! But that confidence lasted all of one day, because not long after the first email, I got another one from Joe saying, wait, wait, that can't be right. He'd thought about it some more and asked around with other experts in the field, and they said, no, those copper nodules are too dense to be pseudomorphs, they are a by-product of the electrowinning process of refining copper from copper ore. In fact, he said, a colleague has a similar nodule collected from just such an industrial operation.
Well, that settles it, right? Since the natural-origins theory has a number of issues, if they have a similar nodule that is known to be artificial, that would be pretty good evidence that mine are artificial too.
I was pretty well convinced that they were artificial at this point. But this new confidence was even shorter-lived. Just a couple of hours later he sent another email giving me permission to quote him on my website, but in this email he mentioned that the "similar" nodule they have has a flat side where the electrode was. Well, if my nodules had a flat side, or any visible point of attachment, I would never have had any doubt that they were electrolytic in nature! While the "natural origin" theory has problems, the "electrolytic" theory has problems too, like why the cuprite core, and how you can form a nodule electrolytically without any asymmetry or point of attachment.
It's not that I can't imagine any way this could be done, it just seems unlikely. The next piece of evidence came in the form of a copper nodule on eBay, now a sample right here. It looks rather similar to these nodules except it's not as round, but it has no flat side and it's claimed to be from electrowinning. I think this pushes the balance of likelihood pretty strongly in the direction of an artificial origin for these nodules.
Personally, I really don't care one way or the other, I just want to know for sure! They are very attractive and interesting objects regardless of whether they are natural or man-made.
In fact, despite not knowing their origin for sure, I've acquired a large quantity to use for trading. If you have any elements you think I'd like, maybe we can make a deal. If you want some of these nuggets and you don't have any elements, contact the source listed below because he's got more he'll sell you.
Source: eBay seller copper_nugget_man
Contributor: Theodore Gray
Acquired: 10 January, 2003
Text Updated: 11 August, 2007
Price: Donated
Size: 0.75"
Purity: 95%
|
|
|
|
|
|
|
Sample from the RGB Set.
The Red Green and Blue company in England sells a very nice element collection in several versions. Max Whitby, the director of the company, very kindly donated a complete set to the periodic table table.
To learn more about the set you can visit my page about element collecting for a general description or the company's website which includes many photographs and pricing details. I have two photographs of each sample from the set: One taken by me and one from the company. You can see photographs of all the samples displayed in a periodic table format: my pictures or their pictures. Or you can see both side-by-side with bigger pictures in numerical order.
The picture on the left was taken by me. Here is the company's version (there is some variation between sets, so the pictures sometimes show different variations of the samples):

Source: Max Whitby of RGB
Contributor: Max Whitby of RGB
Acquired: 25 January, 2003
Text Updated: 11 August, 2007
Price: Donated
Size: 0.2"
Purity: 99.0%
|
|
|
|
|
|
|
Sample from the Everest Set.
Up until the early 1990's a company in Russia sold a periodic table collection with element samples. At some point their American distributor sold off the remaining stock to a man who is now selling them on eBay. The samples (except gases) weigh about 0.25 grams each, and the whole set comes in a very nice wooden box with a printed periodic table in the lid.
To learn more about the set you can visit my page about element collecting for a general description and information about how to buy one, or you can see photographs of all the samples from the set displayed on my website in a periodic table layout or with bigger pictures in numerical order.
Source: Rob Accurso
Contributor: Rob Accurso
Acquired: 7 February, 2003
Text Updated: 29 January, 2009
Price: Donated
Size: 0.2"
Purity: >99%
|
|
|
|
|
|
|
 Electrochemically grown crystal. Electrochemically grown crystal.
This beautiful man-made crystal was grown by electro-deposition from a salt solution. Presumably some kind of electrode was dipped in the solution and copper started growing on it under conditions that encouraged accretion at the ends rather than the sides of the dendrites. I believe these sorts of crystals are an unintended nuisance created during various electroplating operations, but they sure are pretty!
I have a very similar nickel crystal from the same seller.
Source: eBay seller judys-1000s-treasures
Contributor: Theodore Gray
Acquired: 22 February, 2003
Text Updated: 5 March, 2006
Price: $11.50
Size: 2"
Purity: >99%
|
|
|
|
|
|
|
 Electrowinning nodule. Electrowinning nodule.
This nodule is reported to be a result of electrowinning of copper, a process in which electricity is used to reduce copper oxide to copper metal, as a means of extracting the metal from its ore. It's a lot like electroplating, the only difference is that in electroplating your goal is to put copper onto something, while in electrowinning the goal is to get the copper in the first place.
This nodule has almost completely settled the debate about whether my other copper nodules are natural or man made. I had thought that because they showed no visible point where an electrode could have been attached, they couldn't be electrolytic in origin. But this one also has no visible point of attachment, and I have no reason to doubt the claim of its origin (unlike the other nodules, whose claim of origin is unconvincing). So apparently there is an electrolytic process that does not result in a flat side.
Source: eBay seller fullhouse2b3g
Contributor: Theodore Gray
Acquired: 27 April, 2003
Price: $10
Size: 1"
Purity: >95%
|
|
|
|
|
|
|
  Mini periodic table table for Popular Science. Mini periodic table table for Popular Science.
In early 2003 I got an email from an editor at Popular Science column asking if I'd like to write a monthly column. Of course I said yes, and the second article (in the August, 2003 issue) is a story about lost wax casting as I did it when I was fifteen or so. For the photographs in the article I made a few little periodic table tables out of various metals: This one is made of copper, and I also made ones out of zinc, silver, and bismuth.
Source: Marco's Scrap Metal
Contributor: Theodore Gray
Acquired: 27 May, 2003
Price: $1/pound for scrap zinc
Size: 3"
Purity: >90%
|
|
|
|
|
|
|
Shrunken Quarter.
This is a truly baffling object: I have yet to meet anyone who is able to figure out how it was created, before I tell them. It's about the size of a dime, which is unusual since it is in fact a quarter. The shrinking was done using a powerful pulse of magnetic force, with no mechanical contact between the coin and anything doing the squeezing. This is is so amazing I've written an article about it for my monthly Popular Science column. If you want one of these coins, I suggest buying one (see source link) before the article comes out in October 2003, as his production capacity is extremely limited. The source link also has detail information and photos of how the coins are made.
Source: Bert Hickman
Contributor: Bert Hickman
Acquired: 20 June, 2002
Price: Donated
Size: 0.5"
Purity: >90%
Sample Group: Coins
|
|
|
|
|
|
|
  Copper leaf. Copper leaf.
Gold leaf you've probably heard of, but it's possible to get very thin sheets of several different pure metals: Click the Sample Group link below to see all the ones I have, which represent pretty much the complete list of those that are commercially available. (Many, many different mixed alloys are also available in leaf form.).
Leaf like this is so thin it has to be picked up with special Red Squirrel hair brushes (none of that Gray Squirrel crap, mind you) and when it wafts down onto an object it conforms to the shape of the surface, settling in even to details as fine as a fingerprint.
I bought this leaf from a store in New York when I was visiting there with my six-year-old daughter Addie: You can read about our visit here.
Source: Sepp Leaf Products
Contributor: Theodore Gray
Acquired: 11 July, 2003
Price: $65/500 sheets
Size: 3.5"
Purity: >99%
Sample Group: Metal leaf
|
|
|
|
|
|
|
Coin shrinking coil, fragmented.
This is what's left of the copper coil used to magnetically shrink a coin (see above). I got it during the photo shoot for the article I wrote about coin shrinking for my monthly Popular Science column.
Source: Bert Hickman
Contributor: Theodore Gray
Acquired: 17 July, 2003
Price: Donated
Size: 2"
Purity: >95%
Sample Group: Coins
|
|
|
|
|
|
|
Chinese coin.
I got these coins in a gift shop in San Francisco's Chinatown district. I don't know if they are genuine Chinese coins, but I'm pretty sure they are genuine copper, since there's little else they could be made out of that would be any cheaper.
Source: Gift Shop in Chinatown
Contributor: Theodore Gray
Acquired: 24 June, 2003
Text Updated: 29 January, 2009
Price: $2
Size: 1"
Purity: >95%
Sample Group: Coins
|
|
|
|
|
|
|
Link in multi-metal chain.
I had been wondering about how hard it would be to make a multi-part graphite mold with which I could cast chain links around each other. That is, given an existing link, cast a new one interlinked with it. This turns out to be quite do-able: Here is the mold I made (using my drill press as a vertical mill and a round-ended router bit):
 
In case you ever want to try this, I'll give you an important hint: The third link is the real test, not the second one.
Using this mold I have cast a chain out of all the metals I can easily cast. Click the Sample Group link below to see all the links together.
This chain (counted as one sample) is the 600th sample added to my collection.
Source: Hardware Store
Contributor: Theodore Gray
Acquired: 9 August, 2003
Text Updated: 20 February, 2006
Price: $1/pound
Size: 3"
Purity: >99%
Sample Group: Multi-metal Chain
|
|
|
|
|
|
|
Hand-polished 2" ball.
This is a beautiful copper ball, lovingly hand-polished by someone who obviously loves to polish copper balls!
Source: eBay seller dbluett
Contributor: Theodore Gray
Acquired: 3 October, 2003
Price: $19
Size: 2"
Purity: >99%
|
|
|
|
|
|
|
|
|
|
 Museum-grade sample. Museum-grade sample.
In early 2004 Max Whitby and I started selling individual element samples identical or similar to the samples we use in the museum displays we build. These are top-quality samples presented in attractive forms appropriate to the particular element. They are for sale from Max's website and also on eBay where you will find an ever-changing selection of samples (click the link to see the current listings).
This ingot shows two kinds of surfaces, one where the metal cooled in contact with a graphite mold, and the top that was in contact with the air. The top surface is particularly interesting in that it shows the crystal structures that give the surface a wrinkled appearance.
Source: Theodore Gray
Contributor: Theodore Gray
Acquired: 6 March, 2004
Text Updated: 11 August, 2007
Price: See Listing
Size: 4"
Purity: >99%
Sample Group: RGB Samples
|
|
|
|
|
|
|
 Museum-grade sample. Museum-grade sample.
In early 2004 Max Whitby and I started selling individual element samples identical or similar to the samples we use in the museum displays we build. These are top-quality samples presented in attractive forms appropriate to the particular element. They are for sale from Max's website and also on eBay where you will find an ever-changing selection of samples (click the link to see the current listings).
This ingot is designed to show what the surface of the metal looks like when hammered: By comparing with similar hammered ingots of other metals an idea of the hardness and working characteristics of the metal is given. (We use approximately the same hammering force on each ingot, so lead will show much deeper hammer marks than zinc, for example.)
Source: Theodore Gray
Contributor: Theodore Gray
Acquired: 6 March, 2004
Text Updated: 11 August, 2007
Price: See Listing
Size: 4"
Purity: >99%
Sample Group: RGB Samples
|
|
|
|
|
|
|
 Museum-grade sample. Museum-grade sample.
In early 2004 Max Whitby and I started selling individual element samples identical or similar to the samples we use in the museum displays we build. These are top-quality samples presented in attractive forms appropriate to the particular element. They are for sale from Max's website and also on eBay where you will find an ever-changing selection of samples (click the link to see the current listings).
This half-sphere is a particularly attractive paper-weight style sample. The flat surface is similar in appearance to that of the ingot shown above.
Source: Theodore Gray
Contributor: Theodore Gray
Acquired: 6 March, 2004
Text Updated: 11 August, 2007
Price: See Listing
Size: 2"
Purity: >99%
Sample Group: RGB Samples
|
|
|
|
|
|
|
 Native copper with inclusions. Native copper with inclusions.
This is native (naturally occurring) copper that has been squeezed around a bunch of broken rocks and pebbles. It certainly gives one an appreciation of the forces present in the earth to imagine this copper being squeezed around rocks like butter through fingers.
Source: Max Whitby of RGB
Contributor: Max Whitby of RGB
Acquired: 1 February, 2004
Price: Donated
Size: 5"
Purity: >90%
|
|
|
|
|
|
|
Mini element collection.
This is a nice little set from the 1960's. The enclosed price list indicates it cost a few dollars, and the enclosed mercury sample indicates it predates current environmental concerns! Here's a picture of the whole 2-box set:

Source: Blake Ferris
Contributor: Theodore Gray
Acquired: 15 July, 2004
Price: $61/set
Size: 1"
Purity: >98%
|
|
|
|
|
|
|
  Museum-grade sample. Museum-grade sample.
In early 2004 Max Whitby and I started selling individual element samples identical or similar to the samples we use in the museum displays we build. These are top-quality samples presented in attractive forms appropriate to the particular element. They are for sale from Max's website and also on eBay where you will find an ever-changing selection of samples (click the link to see the current listings).
This cast ball is designed to show what the surface of the metal looks like when hammered: By comparing with similar hammered balls of other metals an idea of the hardness and working characteristics of the metal is given. (We use approximately the same hammering force on each ball, so lead will show much deeper hammer marks than zinc, for example.)
I chose this sample to represent its element in my Photographic Periodic Table Poster. The sample photograph includes text exactly as it appears in the poster, which you are encouraged to buy a copy of.

Source: Theodore Gray
Contributor: Theodore Gray
Acquired: 1 August, 2004
Text Updated: 11 August, 2007
Price: See Listing
Size: 2"
Purity: >99%
Sample Group: RGB Samples
|
|
|
|
|
|
|
Lead-free fishing weights.
Environmental concerns have prompted a switch from lead to other metals for fishing sinkers. Click the sample group link below to see what metals have been used
Source: Walmart
Contributor: Theodore Gray
Acquired: 15 July, 2004
Price: $1.50
Size: 0.5"
Purity: >95%
Sample Group: Fishing Weights
|
|
|
|
|
|
|
  Chain mail. Chain mail.
This is hand-made chain mail, meant to be used in jewelry, and made by Mike Lauter who kindly donated some to my collection, including versions made of four different elements (click the Sample Group link below to see the other variations). If you need some chain mail, he'd probably make some for you.
Source: Mike Lauter
Contributor: Mike Lauter
Acquired: 20 May, 2005
Price: Donated
Size: 2"
Purity: >98%
Sample Group: Chain Mail
|
|
|
|
|
|
|
  Penny skeleton. Penny skeleton.
This is a very clever sample. It's a known fact that if you leave a post-1982 penny in strong acid (e.g. muriatic acid) long enough, the zinc core will be dissolved away leaving just the copper cladding. Niels took this one step further by first sanding the penny, removing the copper cladding only from the raised areas. Then when the zinc was dissolved away, the result was an incredibly finely detailed stencil. It's quite delicate: You can see in the rotation video that it flutters a bit from the breeze created by the cooling fans of the fiber optic illuminators used to light it.
Now, if only someone could figure out how to dissolve only the copper, leaving the solid zinc core in place!
Source: Niels Carlson
Contributor: Niels Carlson
Acquired: 20 December, 2005
Text Updated: 9 February, 2006
Price: Donated
Size: 0.5"
Purity: >98%
Sample Group: Coins
|
|
|
|
|
|
|
  Element coin. Element coin.
Dave Hamric sells element samples under the name Metallium. He's developed a line of coins struck out of various common and uncommon metals: They are quite lovely, and very reasonably priced, considering the difficulty of creating some of them.
Here is the back side of this coin (click either picture to see it larger):
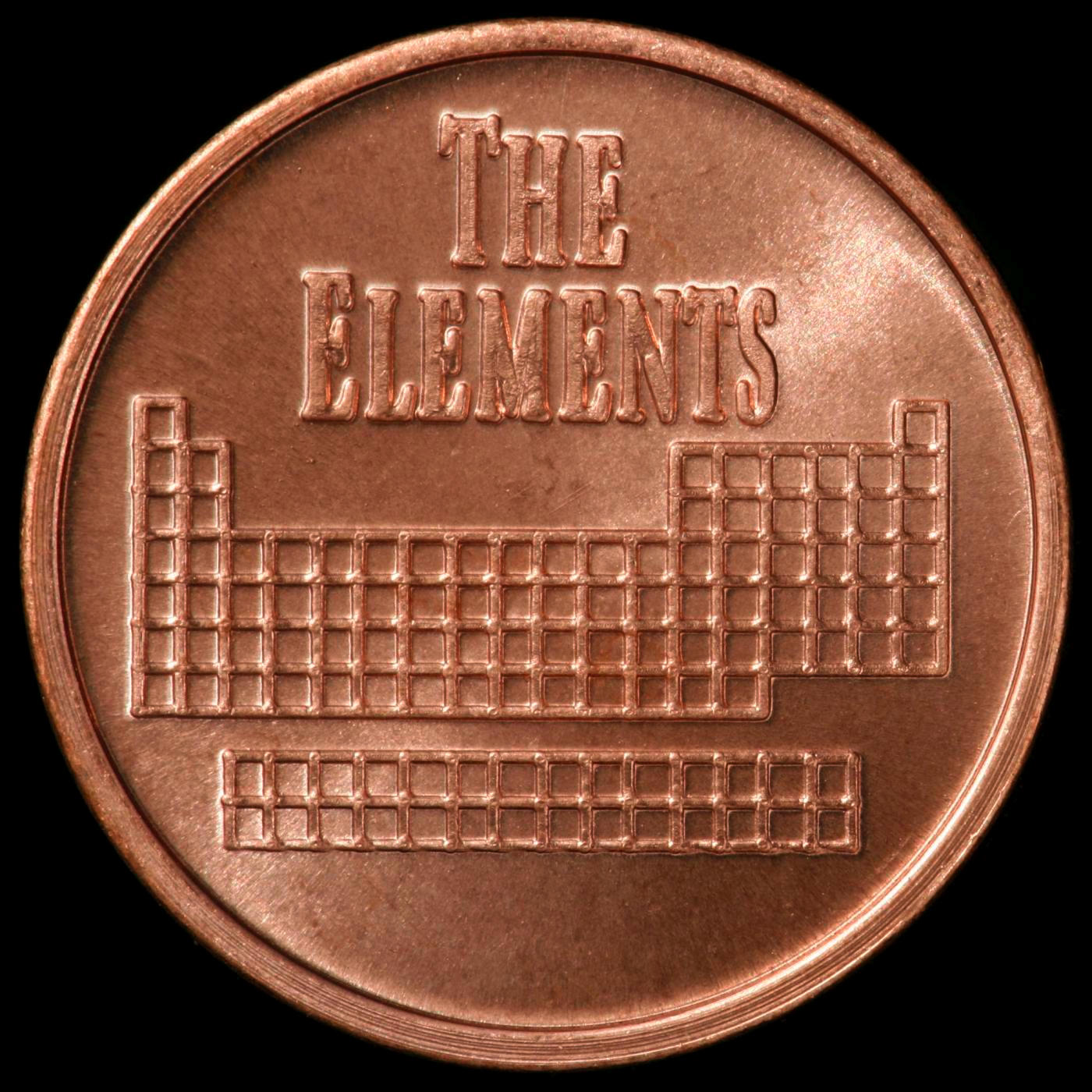
Click the Sample Group link below to see many other coins made of various elements, or click the link to his website above if you want to buy one like this.
Source: Dave Hamric
Contributor: Theodore Gray
Acquired: 1 December, 2006
Text Updated: 14 January, 2007
Price: $5
Size: 0.75"
Purity: >99%
Sample Group: Coins
|
|
|
|
|
|
|
  Heat sink. Heat sink.
Copper has very high thermal conductivity, which means it's good at moving heat from one place to another, useful if you have too much heat in one place (i.e. for cooling applications) or you want to add more heat to something (i.e. in cooking applications). Hence the popularity of copper in heat sinks, like this one intended to cool computer CPU chips, and in cooking pots.
This cool-looking heat sink was sent to me by a kind reader in August 2006, but due to my being very busy trying to finish the next version of Mathematica, I had a huge backlog of samples to photograph and it wasn't until January 2007 that my new photography assistant Nick Mann was able to get to this one. (That is how busy I am: I don't even have time to do my hobby, I have to hire someone to do it for me.) Amazingly, the kind reader emailed me asking when I was ever going to get around to photographing the sample he had kindly contributed, about two hours after Nick had finished processing it. After a five month delay, that's pretty good timing!
Anyway, back to the sample. It has a fairly thick slab of copper on the bottom, the side that will be in contact with the chip. (Typically you would add some heat-conductive cream to the gap to ensure good contact.) Sticking up from the slab are copper fins that carry the heat up into the stream of air generated by the small fan in the back.
Because the thermal conductivity of metals is typically goes up with higher purity, and because this is obviously a good-quality heat sink, I'm going to assume the copper in it is fairly pure.
Source: Dudley Fox
Contributor: Dudley Fox
Acquired: 20 January, 2007
Text Updated: 22 January, 2007
Price: Donated
Size: 5"
Purity: >98%
|
|
|
|
|
|
|
  Antique Japanese coin. Antique Japanese coin.
This is a huge (2.5" diameter), thick coin I got on a recent trip to Japan. It commemorates the visit of the Emperor of Manchukuo to Japan in 1935. (Manchukuo was a puppet state that Japan supported in Manchuria from 1932 to 1945.)
I bought it in a small coin shop, where I had my interpreter ask the shop owner if he had any coins made out of metals that coins are not normally made out of. After some confusion, he looked around and didn't really find anything very interesting in that category, so I asked instead for coins that were at least made out of pure metals, and were in some other way unusual. I'm not a coin collector, so I have no interest in particular dates or mint marks or whatever it is that coin collectors are interested in. This one isn't even a real coin, it's more of a commemorative medallion, known to coin collectors as "exonumia", meaning "not real money so we don't want it".
I like this one because it's so big and heavy, satisfying to hold.
Source: Japan
Contributor: Theodore Gray
Acquired: 12 December, 2006
Text Updated: 12 April, 2009
Price: $10
Size: 2.5"
Purity: >90%
Sample Group: Coins
|
|
|
|
|
|
|
  Nice copper cup. Nice copper cup.
This is a nicely hammered copper cup I got in Japan. It was sold as a health-related object, not surprising since copper is often claimed to have various beneficial effects. Which of course doesn't make much sense when you consider that it doesn't dissolve in water, and even if it did, this cup is lined with some other metal that would prevent you from getting any copper from it. I think it's supposed to be some kind of energy nonsense, people also wear copper bracelets that are said to have similarly non-existent positive effects despite staying entirely outside your body.
Source: Japan
Contributor: Theodore Gray
Acquired: 12 December, 2006
Text Updated: 12 March, 2007
Price: $10
Size: 6"
Purity: >90%
|
|
|
|
|
|
|
  Frangible copper projectile. Frangible copper projectile.
This projectile was described as nearly pure copper with a bit of nylon to hold it together. "Frangible" means it's designed to fragment into thousands of little pieces on impact. This serves two purposes: Increasing damage to the target (i.e. makes a bigger hole) and limiting the danger of the projectile bouncing around in an enclosed space and hitting the shooter.
Source: Derek McKinley
Contributor: Derek McKinley
Acquired: 6 April, 2007
Text Updated: 6 April, 2007
Price: Donated
Size: 0.5"
Purity: >90%
|
|
|
|
|
|
|
  Slab. Slab.
I've long since forgotten where this slab of copper came from, but it's a nice hunk so I decided it should officially be part of my collection, instead of just sitting around unused. Now it can sit around unused but at a much higher aesthetic level.
Source: Unknown
Contributor: Theodore Gray
Acquired: 16 June, 2007
Text Updated: 20 June, 2007
Price: Unknown
Size: 8"
Purity: >95%
|
|
|
|
|
|
|
  Heat sink. Heat sink.
Now this is a nice hunk of copper, the base a good 1/4" thick with lots of copper fins coming up from it. Normally there is a fan mounted directly above the fins blowing air through them, but I've removed it so you can see the copper better. The smooth bottom of the heat sink is pressed onto a large chip with some heat-conductive gel between them: This one is intended for cooling Intel CPUs.
Source: Computer Store
Contributor: Theodore Gray
Acquired: 19 June, 2007
Text Updated: 20 June, 2007
Price: $35
Size: 4"
Purity: >99%
|
|
|
|
|
|
|
  Nice heavy dragon. Nice heavy dragon.
I really like this little brass (i.e. mostly copper) dragon. It came from one of the many tourist trap stores in the Chinatown section of San Francisco.
Source: Gift Shop in Chinatown
Contributor: Theodore Gray
Acquired: 1 July, 2007
Text Updated: 1 July, 2007
Price: $25
Size: 4"
Purity: 50%
|
|
|
|
|
|
|
  Vibrating wire sensor. Vibrating wire sensor.
My grandfather, Armin Wirth, made his fortune inventing weighing scales, machines to weigh everything from ladles full of molten steel to airplanes to powdered sugar. But his enduring contribution to the technology of weighing was the invention of the vibrating wire sensor.
The idea is quite simple: A wire is stretched between two points and caused to vibrate by an alternating current running through it (magnets around the wire have been removed in this photo so you can see the wire). As anyone who has tuned a guitar knows, the resonant frequency of a stretched wire depends on how tightly it is stretched. In other words, the frequency is a measurement of the force stretching the wire, and therein lies a sensor.
The Wirth-Gallo company first started building sensors based on this principle decades ago, but pictured here is a modern incarnation, a compact, relatively inexpensive aluminum unit. You can see the wire stretched across the center near the top of the unit. It's held on either side by a pair of sapphire cylinders squeezed together by conical steel pins pressed into the opposite sides of the two blocks on top.
In use, the force to be measured is applied to a rod inserted through the hole in the thick bottom section and fastened with set screws to the block in the center of the machined aluminum unit. As you can see from the geometry of the device, if you push up on the center block it will deflect upwards, causing the two sets of horizontal supports to angle outwards (going from rectangular to parallelogram shape), which in turn will cause the two blocks holding the wire to be pulled farther apart.
Of course the actual movement is microscopic: The mechanism is designed to transfer force to the wire, not actually move any significant amount. (In fact the whole complex geometry is designed to minimize the negative effects, like metal creep and fatigue, that come about from whatever movement there is.)
If you look at the rotation video you can see how much effort goes into isolating the wire holding block from error forces. For example, elaborately shaped bits of copper connect the ends of the stretched wire to the circuit board.
You may be forgiven for asking, why not just use a Piezo electric crystal force sensor? Piezo sensors are ubiquitous, and they work fine in many applications, but they have limitations, one of which is that it's difficult to keep them accurate under severe operating conditions.
For example, imagine wanting to be able to weigh the garbage collected from each individual house on a collection route, by putting sensors on all four wheels of the garbage truck. (This is not only being done in Europe, in a number of countries it is or soon will be a legal requirement that all garbage collection service by priced by the pound. It tends to make people think a bit more about what they throw away.)
Sensors on the wheels of a garbage truck have what can only be described as a hard life. One minute they are being run over pot holes at high speed, slamming a truck weighing many tons down hard on the sensor. The next minute they are being asked to accurately weigh a few pounds of garbage, by measuring the increases in weight of the multi-ton truck when a new bin is tipped into it.
That's the kind of job for which the vibrating wire sensor has a great advantage over the more delicate Piezo sensors, which tend to drift out of calibration when subject to such rude treatment.
My uncle Johannes Wirth, who took over the business from my grandfather, gave me this sensor when I visited him recently in Zurich (taking my whole family including three smallish children on a two week vacation to London and Switzerland, but that's a whole other story).
Source: Johannes Wirth
Contributor: Johannes Wirth
Acquired: 4 September, 2007
Text Updated: 5 September, 2007
Price: Donated
Size: 2"
Purity: >60%
|
|
|
|
|
|
|
  Copper-plated video iPod Nano. Copper-plated video iPod Nano.
This sample is the result of an article I wrote for the December, 2007 issue of Popular Science Magazine, for which I write a monthly column. Follow either of those links to find the article, or if it's between November 10th and December 10th, 2007 when you're reading this, head out to your local news stand and buy a copy of the magazine.
Basically the idea was to find a creative way to deal with the ridiculously shiny back side of Apple's otherwise flawless iPod line. The backs are so perfectly shiny that they are ruined the instant you touch them, with fingerprints and scratches appearing within seconds of purchase. My solution was to sandpaper the back into a pleasing brushed finish, then replace the Apple logo I'd sanded of with an electro-plated copper periodic table. Read the article to see how this was done.
Source: Theodore Gray
Contributor: Theodore Gray
Acquired: 9 November, 2007
Text Updated: 21 November, 2007
Price: $200
Size: 2.8"
Purity: >95%
|
|
|
|
|
|
|
|
|
|
  Copper scrap. Copper scrap.
My assistant Nick Mann has done a fair bit of photography work for Mervis Industries, an industrial metal recycler. They even sent him to Mexico to photograph their operations there, which is where he found this pretty piece of copper scrap. My guess is it's the left over bits from which electrical plug blades have been stamped out, but that's just a guess.
Source: Nick Mann
Contributor: Nick Mann
Acquired: 20 November, 2007
Text Updated: 21 November, 2007
Price: Donated
Size: 2"
Purity: >99%
|
|
|
|
|
|
|
  Copper Bermuda pig coin. Copper Bermuda pig coin.
Here in America we are used to finding Canadian pennies and quarters in our change. They look similar enough that they often pass as the genuine article, and people used to make fun of them for being worth a good bit less than the real American version. Well that is no more, the Canadian dollar is now worth more than the pathetic US version, and I suppose it's inevitable that those people up there are now laughing at our worthless pennies and quarters when they find them in their pockets.
But this is not an Canadian penny, so I don't know why I'm going on about Canada. This is something much stranger to have pop up in your pocket: A penny from Bermuda. With a pig on the back! I mean, honestly, what sort of country puts a pig on the back of any kind of coin, even just a penny? I admit it's a very handsome pig, but still, in my forty some years I have never seen the like of it for sheer silliness in coinage. Is Bermuda even a real country?
Coin collector Joshua Holman provided the following clarifications:There is a story behind the pig. When Bermuda was first settled by the English, pigs were the only food on the island. Also the pig is a tribute to the original money issued by the settlers (it had pigs on it as well). The motif on the penny reflects these things. On the page, you also wonder "Is Bermuda even a real country?"
The answer to that is no. Bermuda is is "overseas territory" of the United Kingdom (i.e., a colony).
Source: My Pocket
Contributor: Theodore Gray
Acquired: 20 November, 2007
Text Updated: 18 December, 2007
Price: $0.01
Size: 0.5"
Purity: >90%
Sample Group: Coins
|
|
|
|
|
|
|
  Copper vacuum gasket. Copper vacuum gasket.
This ring is a used vacuum gasket. A gasket is a soft material placed between two mating surfaces with the idea that, being soft, it will deform itself to fill in any small gaps or irregularities in the surfaces, creating an air-tight seal. A common rubber washer is an example of a gasket, and indeed most gaskets you see in common use are made of rubber or rubber-like materials.
But for high-vacuum applications rubber or plastic is completely unsuitable. They may be air-tight according to ordinary, every-day standards, but they are quite porous by the standards of high vacuum work. Any sort of organic matter absorbs gas that comes back out when it's placed under vacuum, and allows oxygen, water vapor, and other gases to pass through slowly.
Metal makes a much better gasket material because it really is completely air-tight. Copper may not sound very soft, but compared to steel it is. See the groove in the center of the ring? That's created by a steel edge when the joint is assembled: By squeezing the joint together so tight that the two parts being joined actually dig into the copper gasket, a completely tight joint is created. It also means the gasket can be used only once, which is why I have several of these: After one use, they are junk.
Source: Max Cane
Contributor: Max Cane
Acquired: 23 December, 2007
Text Updated: 29 January, 2009
Price: Donated
Size: 2.5"
Purity: >95%
|
|
|
|
|
|
|
  Copper leaf in bottle. Copper leaf in bottle.
This is a little souvenir bottle of copper leaf from a gift shop at the Keystone sky resort in Colorado (nice place, by the way). Silver mining was big in that area, with several towns (Silverthorn, Silverplume, etc) named for it. I'm not sure, but given that they are in the same column in the periodic table and therefore share chemical properties, it stands to reason that copper and silver would be found together in the same mines. The amount of copper in this bottle is microscopic because it, like gold, can be hammered into extremely thin leaf form. I have similar bottles listed under gold and silver.
Source: Keystone Resort
Contributor: Theodore Gray
Acquired: 8 March, 2008
Text Updated: 8 March, 2008
Price: $8
Size: 2"
Purity: 95%
|
|
|
|
|
|
|
  Interesting melted shape of copper. Interesting melted shape of copper.
Description supplied by the source:
This is a good sample of several possible appearances for copper- the top is wire-brushed, if memory serves, the bottom is the as-cast surface that was up against the also-copper crucible, and the side shows the internal crystalline structure. I melted this particular piece from 4N5 pure melt stock in a shallow crucible: It's just a nice looking sample.
Source: Ethan Currens
Contributor: Ethan Currens
Acquired: 21 March, 2008
Text Updated: 21 March, 2008
Price: Anonymous
Size: 3"
Purity: 99.99%
|
|
|
|
|
|
|
  Copper turtle. Copper turtle.
This pretty little copper and glass turtle was picked up in a gift shop by my photographer assistant Nick Mann in Philadelphia.
Source: Nick Mann
Contributor: Nick Mann
Acquired: 14 June, 2008
Text Updated: 14 June, 2008
Price: Donated
Size: 4"
Purity: >95%
|
|
|
|
|
|
|
|
|
|
|
|
|
  Copper "ingot". Copper "ingot".
Described as an "ingot" by the seller, this is actually a piece of rolled copper bar cut to length. It was being promoted as being suitable for investment purposes, but while the price of copper has gone up a lot, it's really not a commodity you can practically invest in in its physical form: The cost of moving it around would swamp any likely profit.
Source: eBay seller dfried18
Contributor: Theodore Gray
Acquired: 30 September, 2008
Text Updated: 1 October, 2008
Price: $1.36
Size: 4"
Purity: 99.9%
|
|
|
|
|
|
|
  Metal salesmans sample. Metal salesmans sample.
From the eBay item description, which pretty much sums up what this item is:This auction is for a very unusual, what I think would be called a Salesman Sample, of 5 exotic metal disks. The brown plastic case that measures 2.50 in. by 6 in., was received by my Father when he visited the Kawecki Berylco Industries in the 1970's. The company was bought by Cabot Corp. in the late 70's. The disks measure 1.25 in. dia. by .1in. thick. According to the description in the case the 5 metals are Beryllium, Lockalloy, Beryllium Copper, Columbium [an older name for niobium], Tantalum. This is the beryllium copper sample. Click the Source link to see the samples all together, and here's a picture of the whole set:

Source: eBay seller jacav111
Contributor: Theodore Gray
Acquired: 27 December, 2008
Text Updated: 28 December, 2008
Price: $134/set
Size: 1.25"
Purity: 97%
|
|
|
|
|
|
|
|
|
|
  Copper hair brush. Copper hair brush.
I'm going to give them the benefit of the doubt and say that the tines on this brush are actually made of copper, even though the copper-colored edge and ring are just plastic. Why anyone would want to pay extra for a brush with copper tines I don't know, the health benefits listed on the box are obviously some kind of joke designed to amuse shoppers on an otherwise dull day.
Source: Walmart
Contributor: Theodore Gray
Acquired: 9 February, 2009
Text Updated: 8 February, 2009
Price: $8
Size: 4"
Purity: 99%
|
|
|
|
|
|
|
  Old copper powder. Old copper powder.
This high-purity copper powder is at least 20 years old, or so says Ethan. The color is characteristic of copper minus its metallic sheen (due to the fineness of the powder, not oxidation).
Source: Ethan Currens
Contributor: Theodore Gray
Acquired: 9 February, 2009
Text Updated: 8 February, 2009
Price: Trade
Size: 1"
Purity: 99.999%
|
|
|
|
|
|
|
  Copper-plated BBs. Copper-plated BBs.
There are pretty copper-plated BBs for sort-of toy guns. Sort of because they certainly aren't real guns, but they're not exactly toys either, at least not by modern toy safety standards.
Source: Walmart
Contributor: Theodore Gray
Acquired: 28 February, 2009
Text Updated: 1 March, 2009
Price: $5
Size: 0.125"
Purity: 95%
|
|
|
|
|
|
|
  Copper scrubbing pad. Copper scrubbing pad.
This is a copper-plated scouring pad. I'm not sure what the copper is plated over, but I would guess iron.
Source: Walmart
Contributor: Theodore Gray
Acquired: 28 February, 2009
Text Updated: 1 March, 2009
Price: $2
Size: 3"
Purity: 1%
|
|
|
|
|
|
|
  Brass parting tool by Kobalt. Brass parting tool by Kobalt.
This is quite simply the most beautiful parting tool yet created by the hand of man. Absolutely lovely. Oh, a parting tool? That's the tool you use to create those rounded grooves you see in concrete surfaces. The grooves are there so that when the concrete cracks, and it will crack, the cracks occur at the bottom of the grooves where you don't see them. This tool is slid over the surface of the concrete after it has started to set up, but is still workable.
What a joy it must be to use such a profoundly beautiful piece of metal on the job, and what pride there must be in creating it. Yes, it's expensive.
Source: Hardware Store
Contributor: Theodore Gray
Acquired: 28 February, 2009
Text Updated: 1 March, 2009
Price: $30
Size: 6"
Purity: 80%
|
|
|
|
|
|
|
|
|
|
  Medieval bullet mold. Medieval bullet mold.
According to the eBay seller:Circa 15th-17th century AD. Medieval bronze bullet die. The die was used to cast bullets for muskets. Beautiful green patina. Length: 23 mm. Well it's obviously only half of the mold, you can see how it has index pins meant to fit with the other half to form a complete sphere mold. I'm a little skeptical about the dates, it looks a tad too sharply cast to me, but what do I know about ancient brass.
Source: eBay seller ancientcaesar
Contributor: Theodore Gray
Acquired: 2 April, 2009
Text Updated: 7 April, 2009
Price: $10
Size: 1"
Purity: <80%
|
|
|
|
|
|
|
|
|
|
  Lovely copper chain. Lovely copper chain.
From the source:This is Half-Persian 4-in-1 weave (which has nothing to do with Persia) made from electrical copper wire. This modern weave is quite popular for jewelry, often used as simple chains.
Source: Eric Winter
Contributor: Eric Winter
Acquired: 15 April, 2009
Text Updated: 16 April, 2009
Price: Donated
Size: 3"
Purity: 99%
|
|
|
|
|
|
|
|
|
|
|
|
|
  Replica copper coin. Replica copper coin.
I believe I got this pretty little cross from a silversmith at a Renaissance Fair held at Rockome Gardens (see source link). I believe he said it was a replica of an early American coin, but sadly I don't remember the details.
Source: Rockome Gardens
Contributor: Theodore Gray
Acquired: 28 June, 2009
Text Updated: 29 June, 2009
Price: $10
Size: 1"
Purity: >90%
|
|
|
|
|
|
|
  Copper sulfate drain cleaner. Copper sulfate drain cleaner.
For some reason copper sulfate is sold for use in keeping drains clear. I think it's supposed to inhibit the growth of roots that may be working they way into underground clay drains.
This brand has very pretty, and fairly large, crystals.
Source: Farm & Fleet
Contributor: Theodore Gray
Acquired: 28 June, 2009
Text Updated: 28 June, 2009
Price: $8
Size: 2"
Purity: 40%
|
|
|
|
|
|
|
  Copper sulfate drain cleaner. Copper sulfate drain cleaner.
For some reason copper sulfate is sold for use in keeping drains clear. I think it's supposed to inhibit the growth of roots that may be working they way into underground clay drains.
This brand consists of fine needle-like crystals.
Source: Farm & Fleet
Contributor: Theodore Gray
Acquired: 28 June, 2009
Text Updated: 28 June, 2009
Price: $8
Size: 2"
Purity: 40%
|
|
|
|
|
|
|
|
|
|
  Copper ring. Copper ring.
A nice decorated copper ring.
Source: Unknown
Contributor: Unknown
Acquired: 13 January, 2010
Text Updated: 13 January, 2010
Price: Donated
Size: 0.75"
Purity: >95%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Michigan Native Float Copper.
To quote from the tag that came with this sample:"Float" copper is found throughout the "Copper Country" of Michigan and many nearby states. Glacial movements thousands of years ago altered the geologic deposits of the native copper by tearing and scouring the land. Copper, along with other rocks, gravel, and sand were constantly tumbled and deposited over large areas of the Upper Midwestern United States.
Exposure to the soils, water, and air has oxidized the copper surface a bright green. These specimens with the natural oxidation color provide a good contrast to the polished copper surface of the cross-sections. I wouldn't exactly call the oxidized surfaces bright green, but they are distinctly copper-green.
Source: eBay seller ldcurtis2002
Contributor: Theodore Gray
Acquired: 3 June, 2003
Price: $2.85
Size: 3"
Composition: Cu
|
|
|
|
|
|
|
  Manganese Nodule. Manganese Nodule.
Do you remember when manganese nodules were going to be the next great gold rush? When a great new natural resource was going to be unleashed just as soon as someone figured out how to dredge them up from the incredibly deep ocean? Did you ever wonder if there might not be some in shallower water?
Well, guess what: The whole thing was a complete fabrication. The CIA wanted to recover a Soviet submarine that had gone down in very, very deep water in the Pacific, and they needed a cover story because they knew that there was no way they could build and deploy the highly specialized kind of ship required to recover something from such great depth without the Russians (who knew exactly where their submarine had gone down) figuring out that something was up.
So they enlisted Howard Hughes, the richest man in the world at the time and a notable nutcase, to pretend that he thought these manganese nodules, which just happened to exist only at great depths, were the next big thing. He built a large, specialized deep sea recovery ship, the Glomar Explorer, and sent it to find, um, um, manganese nodules, that's right, we're looking for manganese nodules.
They actually did find the Soviet submarine and were able to recover parts of it. Eventually people forgot about the manganese nodules.
If you don't believe me, read this report on the subject:
http://www.fas.org/irp/program/collect/jennifer.htm
This particular nodule was recovered from 5100m of water in the central pacific by the MS Valdiva working for the Metallgesellschaft AG, Frankfurt am Main. I wonder if they thought they were going to get rich.
Source: eBay seller mitryrock
Contributor: Theodore Gray
Acquired: 3 June, 2003
Price: $20.50
Size: 1"
Composition: MnNiCuCo
|
|
|
|
|
|
|
 Native Copper, Caledonia Mine. Native Copper, Caledonia Mine.
This is a heavy lump of naturally occurring copper from the Caledonia Mine, Ontonagon County, Michigan. Like several of the dozen samples I got from the same source, this one has what look like impressions of pebbles, as it was compressed around them at some point in its history.
Source: Larry Curtis
Contributor: Theodore Gray
Acquired: 10 June, 2003
Price: $2.50
Size: 2"
Composition: Cu
|
|
|
|
|
|
|
63 pound solid brass cylinder.
The first question is, "is it hollow?", followed rapidly by "is it glued down?". No, it's solid and no, it's just really heavy. I got this as a dirty, rough cutoff at Marco's scrap metal, but it cleaned up pretty nicely with a combination of power and hand sanding to 600 grit, followed by buffing with some polishing compounds.
The density of brass is really not that much less than lead, which makes this cylinder a nice complement to my lead doorstop.
Source: Marco's Scrap Metal
Contributor: Theodore Gray
Acquired: 21 August, 2003
Price: $35
Size: 10"
Composition: CuZn
|
|
|
|
|
|
|
Copper Petrified Wood.
The tag that came with sample reads as follows:Petrified wood from the Nacimiento Mine
Replacement minerals could include Malachite, Chalcocite, Azurite, Cuprite, Covellite, Digenite, Bornite, Plancheite, Iron Oxide, Pseudomalachite, Pyrite, Cubanite, Brochanite, Antlerite, Elemental Copper, Stromeyerite. But it's the copper we're interested in here! The green color indicates that copper is a major constituent of whichever minerals make up this sample. In fact, I traded this sample for a few of my strange copper nodules.
Source: Calvin Webb
Contributor: Calvin Webb
Acquired: 1 September, 2003
Price: Donated
Size: 2"
Composition: Cu
|
|
|
|
|
|
|
  Torbernite. Torbernite.
Torbernite is a lovely, lovely green color (I would guess from the copper). It's also quite radioactive, from the uranium content, and even more so from the mixture of uranium decay products that have built up in it over millions of years.
Source: eBay seller migalf1
Contributor: Theodore Gray
Acquired: 3 June, 2005
Price: $27
Size: 1.5"
Composition: Cu(UO2)2(PO4)2.8-12H2O
|
|
|
|
|
|
|
|
|
|
|
|
|
  Random native copper. Random native copper.
If I could remember where this came from, I'd tell you more about it. One of the hazards of getting way behind in cataloging samples is that when it comes time to describe them, you have no idea what to say.
Source: Unknown
Contributor: Theodore Gray
Acquired: 1 January, 2006
Text Updated: 5 December, 2006
Price: $2.50
Size: 1"
Composition: Cu
|
|
|
|
|
|
|
  Brass shell casings. Brass shell casings.
This is a collection of assorted shell casings made of brass (a copper-zinc alloy). In use, casings like this would be filled with gunpowder or other propellent, with a projectile (bullet) fastened in the open end.
Source: Derek McKinley
Contributor: Derek McKinley
Acquired: 13 February, 2007
Text Updated: 14 February, 2007
Price: Donated
Size: 2"
Composition: CuZn
|
|
|
|
|
|
|
  Brass split ring. Brass split ring.
The Boeing aircraft company operates a wonderful surplus store in Kent, Washington (near Seattle). I have no idea why they made, and then had second thoughts about using, this lovely huge, heavy, split ring of brass, but they did, and it ended up in their surplus store. It looks like something you could make a magnet out of, if it were iron instead of brass.
Source: Boeing Surplus Store
Contributor: Theodore Gray
Acquired: 11 August, 2007
Text Updated: 11 August, 2007
Price: $15
Size: 7"
Composition: CuZn
|
|
|
|
|
|
|
  Insanely expensive knife. Insanely expensive knife.
This is a really, really nice knife, but honestly, I don't know what I was thinking. I plead temporary insanity. Not that I regret it or anything, this is just too good an, um, element sample to pass up. A bargain at half the price.
Anyway, the blade is the most remarkable thing about it, with the handle running a close second. The blade is patterned Damascus steel: What looks like an etched design on the surface actually goes all the way through the thickness of the blade. You can see other examples of Damascus steel on my site, but notice how they all have random wavy patterns.
Damascus steel is made by taking a sheet of steel, folding it over, heating it in a forge, then hammering it until it's as thin as it was before being folded. Then it's folded again, heated up, hammered out, etc, until it's been folded many times. Because the surface is being oxidized and carbonized from the heat at each stage, you end up with dozens or hundreds of alternating layers of bright steel and hard, dark, carbonized steel. For hundreds of years this was the finest, sharpest, and hardest steel available.
But look at the blade: The pattern is anything but random. In fact, it looks a lot like a 3D plot from Mathematica, which is what first attracted me to the knife. This is Damascus steel where the sequence of folds has been carefully designed to result in a particular pattern, not just a random waves. It's still folded and hammered by hand, but according to a very particular sequence designed, I am told, by computer (thought not with Mathematica, so far as I know).
For the blade it's the pattern that makes it special, but for the handle it's the materials. The handle is also made with a Damascus-style folding technique, but instead of folding steel onto itself, it's made by folding together alternating layers of niobium and copper. Oooo, a niobium-handled knife, now that really gets me going. Looking closely, you can see the reddish copper inclusions clearly within the silver colored niobium metal. (There's also an inlay of black pearl, but this is of no interest to an element collector.)
Adding a further level of interest, among all my dangerous and/or radioactive samples, this is the only one that it might actually be illegal for me to own. It is, you see, a fully spring-loaded switchblade. Push a button and the blade snaps out under its own power. According to my reading of the federal switchblade law there's only one way that I could legally own this knife, and that's by cutting off my arm. Yes really, let me quote from United States Code, Title 15, chapter 29, section 1244 Exceptions:(4) the possession, and transportation upon his person, of any switchblade knife with a blade three inches or less in length by any individual who has only one arm. Since the blade is less than 3" long, the solution is simple.
Or maybe not so simple, because though with one arm I would be allowed to possess and carry it according to Federal law, according to Illinois state law I would still be in hot water. In California, where I got the knife, it is legal to possess such a knife even if you have two arms, but not to carry it on your person, regardless of your arm count. So if anyone asks, I keep the knife in California, but so far have resisted the temptation to use it to cut off my arm.
Source: California Knife Store
Contributor: Theodore Gray
Acquired: 4 September, 2007
Text Updated: 6 September, 2007
Price: $3000
Size: 3.5"
Composition: FeNbCu
|
|
|
|
|
|
|
  Electromagnetic Sensor. Electromagnetic Sensor.
Ah, this brings back memories. I made this thing some time in high school: It's supposed to be a general purpose "microphone" for electric or magnetic fields or vibrating metal parts. I turned the handle on a little toy wood lathe, and got a coil of fine wire from a small electric motor. Behind the coil are a couple of permanent magnets from Radio Shack. If you connect it (using the RCA jack at the base of the handle) to an audio amplifier you can actually hear things when you hold it near something that's producing oscillating fields (e.g. a speaker, electric appliance, etc). The idea behind the permanent magnets is to make it work with any vibrating metal, not just electrically-active objects, but that part never really worked as well as I'd hoped.
Source: Theodore Gray
Contributor: Theodore Gray
Acquired: 23 December, 2007
Text Updated: 23 December, 2007
Price: Priceless
Size: 5"
Composition: CuFe+C(H2O)
|
|
|
|
|
|
|
  Native Copper from Germany. Native Copper from Germany.
My assistant Nick brought this sample of native (naturally occurring copper) back from his trip to Germany.
Source: Germany
Contributor: nick
Acquired: 26 September, 2008
Text Updated: 27 September, 2008
Price: Donated
Size: 2"
Composition: Cu
|
|
|
|
|
|
|
|
|
|
|
|
|
  Azurite. Azurite.
Description from the source:
Azurite (Cu+23 (CO3)2 (OH)2 mon.), Ajo, Pima Co., Arizona, USA. Well definited crystals on matrix. 1,8x1,5x1 cm; 8 g.
Source: Simone Citon
Contributor: John Gray
Acquired: 30 September, 2008
Text Updated: 1 October, 2008
Price: Trade
Size: 0.7"
Composition: Cu+23(CO3)2(OH)2
|
|
|
|
|
|
|
  Stannite. Stannite.
Description from the source:
Stannite (Cu2 Fe Sn S4 tet.), San Jose` Mine, Oruro, Bolivia. Yellowish masses or pseudocrystals with prismatic dark gray Zinkenite. 1,6x1,4x1,2 cm; 5 g.
Source: Simone Citon
Contributor: John Gray
Acquired: 30 September, 2008
Text Updated: 1 October, 2008
Price: Trade
Size: 0.6"
Composition: Cu2FeSnS4
|
|
|
|
|
|
|
  Carrollite. Carrollite.
Description from the source:
Carrollite (Cu (Co Ni)2 S4 cub.), Kamoya II Mine, Shaba, Rep. Dem. of Congo. Perfect crystal on matrix. 5x4x3,2 cm; 87 g.
Source: Simone Citon
Contributor: John Gray
Acquired: 30 September, 2008
Text Updated: 1 October, 2008
Price: Trade
Size: 2"
Composition: Cu(CoNi)2S4
|
|
|
|
|
|
|
  Malachite. Malachite.
Description from the source:
Malachite (Cu+22 (CO3) (OH)2 mon.), Germany. Acicular crystals on limonitic matrix. 2,5x1,5x1,2 cm; 12 g with box.
Source: Simone Citon
Contributor: John Gray
Acquired: 14 October, 2008
Text Updated: 14 October, 2008
Price: Trade
Size: 1"
Composition: Cu+22(CO3)(OH)2
|
|
|
|
|
|
|
  Atacamite. Atacamite.
Description from the source:
Atacamite (Cu+2 2 Cl (OH)3 orth.), La Farola Mine, Tierra Amarilla, Atacama, Chile. Dark green acicular crystals on matrix. 6x4x2 cm; 52 g.
Source: Simone Citon
Contributor: John Gray
Acquired: 26 October, 2008
Text Updated: 28 April, 2009
Price: Trade
Size: 2.35"
Composition: Cu2[(OH)3|Cl]
|
|
|
|
|
|
|
  Cornetite. Cornetite.
Description from the source:
Cornetite (Cu+23(PO4) (OH)3 orth.), Shaba, Rep. Dem. of Congo. Rare crystal sections on matrix. 3,2x2x0,8 cm; 4 g.
Source: Simone Citon
Contributor: John Gray
Acquired: 30 October, 2008
Text Updated: 31 October, 2008
Price: Trade
Size: 1.25"
Composition: Cu+23(PO4) (OH)3
|
|
|
|
|
|
|
|
|
|
  Superconducting powder. Superconducting powder.
This is a small vial of superconducting yttrium-barium-copper-oxide powder. Normally this type of material is seen pressed into pellets that can be used to levitate magnets or perform other superconducting experiments. Background information from the source:Yttrium barium copper oxide was discovered in the late 1980's as being the first known substance to exhibit superconductivity above 77 Kelvin (-321\272F, -195C) outside of a mind-meltingly strong magnetic field. In the grand scheme of things, 77 Kelvin is a completely random value--it has no greater cosmic meaning, it's not an ultimate limit that contradicts some obscure physics theory, nothing like that. Rather, 77 Kelvin is the boiling point of nitrogen. The significance is simply that with the discovery of this material, superconductivity was a phenomenon that suddenly emerged from the realm of "laboratory curiosity" into the wide bright world of "industrially and economically feasible," using liquid nitrogen as a coolant. Previously, liquid hydrogen or liquid helium were necessary to access the temperatures at which superconductivity could be observed (outside of that frog-levitatingly strong magnetic field), both of which are at least an order of magnitude more expensive than liquid nitrogen.
The next breakthrough of the same significance would be the discovery of superconductivity at 194 Kelvin, the temperature of dry ice, which at the time of this writing, is not too far from realization (the current recordholder is 138 Kelvin, using a precise composition of Mercury Thallium Barium Calcium Copper Oxide...nice and toxic too). As a result, it is one of the most popular fields in materials and physics research today, which leads to samples such as this one.
However, the superconductive properties of YBCO depend intimately on the exact composition, which is never stoichiometric (integer values for the elemental subscripts). At the most basic level, special methods of synthesizing and altering this compound are used to add and subtract oxygens, which changes the valence state of copper so that different valence states are scattered through the material. Interestingly, the actual cause and mechanism of superconductivity is extremely complicated, and not known precisely. But researchers in a laboratory would likely take this sample, and use it in either a oxidizing or deoxidizing process to create exactly the composition they desired for whatever phenomena they were investigating.
Gentlemen, start your measurements!
Source: Anonymous
Contributor: Theodore Gray
Acquired: 27 December, 2008
Text Updated: 1 March, 2009
Price: Anonymous
Size: 1"
Composition: YBa2Cu3O7
|
|
|
|
|
|
|
Metal salesman's sample pouch.
From the eBay item description, which pretty much sums up what this item is:This auction is for a very unusual, what I think would be called a Salesman Sample, of 5 exotic metal disks. The brown plastic case that measures 2.50 in. by 6 in., was received by my Father when he visited the Kawecki Berylco Industries in the 1970's. The company was bought by Cabot Corp. in the late 70's. The disks measure 1.25 in. dia. by .1in. thick. According to the description in the case the 5 metals are Beryllium, Lockalloy, Beryllium Copper, Columbium [an older name for niobium], Tantalum. The individual disks are listed under their respective elements (beryllium for lockalloy, copper for beryllium copper).
Source: eBay seller jacav111
Contributor: Theodore Gray
Acquired: 27 December, 2008
Text Updated: 28 December, 2008
Price: $134
Size: 6"
Composition: BeAlCuNbTa
|
|
|
|
|
|
|
  Lab-created copper nodules. Lab-created copper nodules.
Description from the source:
Copper (Cu cub.), Laboratory product, but nice, as a grapes bunch. 2x1,5x1,2 cm; 8 g.
Source: Simone Citon
Contributor: John Gray
Acquired: 10 January, 2009
Text Updated: 10 January, 2009
Price: Trade
Size: 0.75"
Composition: Cu
|
|
|
|
|
|
|
  Large Petrified Wood. Large Petrified Wood.
This is a large, heavy piece of petrified wood. I'm listing it under copper because it's greenish in color, which usually indicates copper content. There's probably more silica and calcium than copper, but you could say that about nearly any rock.
Source: Rockome Gardens
Contributor: Theodore Gray
Acquired: 29 January, 2009
Text Updated: 29 January, 2009
Price: $10
Size: 10"
Composition: Cu
|
|
|
|
|
|
|
  Native Copper. Native Copper.
Description from the source:
Copper (Cu cub.), Inca de Oro, Copiapo`, Chile. Pseudo crystal cluster from an historic mine. 2,5x2x0,4 cm; 6 g.
Source: Simone Citon
Contributor: John Gray
Acquired: 28 January, 2009
Text Updated: 29 January, 2009
Price: Trade
Size: 1"
Composition: Cu
|
|
|
|
|
|
|
  Renierite. Renierite.
Description from the source:
Renierite ((Cu,Zn)11(Ge,As)2Fe4S16 tetr.), Kipushi, Shaba, Dem. Rep. of Congo. Perfect example, with brown-orange-reddish cristalline masses. 1,5x1,2x1 cm; 3 g.
Source: Simone Citon
Contributor: John Gray
Acquired: 28 January, 2009
Text Updated: 29 January, 2009
Price: Trade
Size: 0.6"
Composition: (Cu,Zn)11(Ge,As)2Fe4S16
|
|
|
|
|
|
|
  Compact flash card hard drive. Compact flash card hard drive.
This is just crazy. When I first heard about these things my jaw literally dropped (not literally). They are obsolete now, having been hopelessly beaten by solid state flash memory, but in their day they were the highest capacity compact memory cards available, up to 8GB by 2008 (by which time 64GB flash memory cards were available).
And they are mechanical hard disk drives. Let me remind you of the dimensions of a compact flash card (type II): 1.4" x 1.7" x 0.2" (36.4mm x 42.8mm x 5mm). The platter in this drive is about 1" (2.5cm) in diameter. It's just crazy small. There's an electric motor spinning the platter, an electro-magnet that moves the read-write heads back and forth, the whole works, plus of course all the control and interface electronics, packing into no space.
I stand in awe of this device.
The platters are aluminum, the electronics are silicon, the wiring is copper, the magnets are neodymium iron boron, and the magnetic coating is iron and cobalt based.
Source: Electronics Store
Contributor: Theodore Gray
Acquired: 28 February, 2009
Text Updated: 1 March, 2009
Price: $100
Size: 1.75"
Composition: AlSiCuCoFeNdB
|
|
|
|
|
|
|
  Density Set. Density Set.
A cute little set of six cubes made from different metals, used to show students how different their densities can be. For cost reasons these sets rarely contain any really dense elements, such as tungsten, which is a pitty since students thus come away with the idea that lead is the densest metal, which is far from the truth. Osmium is twice as dense, and tungsten a good 75% more dense.
Source: Educational Innovations
Contributor: Theodore Gray
Acquired: 11 March, 2009
Text Updated: 12 March, 2009
Price: $20
Size: 1"
Composition: PbCuFeZnAlZn
|
|
|
|
|
|
|
  Metatorbernite. Metatorbernite.
Description from the source:
Metatorbernite ( Cu+2 (UO2)2 (PO4)2x8H2O tet.), Monte Painter, Australia. Green laminar crystals on limonitic, rich in oxides matrix. 6,5x5x3 cm; 87 g.
Source: Simone Citon
Contributor: John Gray
Acquired: 11 March, 2009
Text Updated: 12 March, 2009
Price: Trade
Size: 2.5"
Composition: Cu(UO2)2(PO4)2.8H2O
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
  Copper sulfate crystal. Copper sulfate crystal.
An individual crystal of copper sulfate from a bottle of root killer (designed to poured down drains to get rid of roots growing into drain lines).
Source: Farm & Fleet
Contributor: Theodore Gray
Acquired: 13 January, 2010
Text Updated: 13 January, 2010
Price: $0.01
Size: 0.25"
Composition: CuSO4
|
|
|
|
|
|
|
  Copper sulfate crystal. Copper sulfate crystal.
An individual crystal of copper sulfate from a bottle of root killer (designed to poured down drains to get rid of roots growing into drain lines).
Source: Farm & Fleet
Contributor: Theodore Gray
Acquired: 13 January, 2010
Text Updated: 13 January, 2010
Price: $0.01
Size: 0.25"
Composition: CuSO4
|
|
|
|
|
|
|
  Copper sulfate crystal. Copper sulfate crystal.
An individual crystal of copper sulfate from a bottle of root killer (designed to poured down drains to get rid of roots growing into drain lines).
Source: Farm & Fleet
Contributor: Theodore Gray
Acquired: 13 January, 2010
Text Updated: 13 January, 2010
Price: $0.01
Size: 0.25"
Composition: CuSO4
|
|
|
|
|
|
|
  Davy Lamp. Davy Lamp.
An antique "Davy lamp", named after Sir Humphry Davy and used by miners before the invention of electric lights. The wire mesh, against all odds, prevents the flame from igniting flammable gases surrounding the lamp in a mine.
Source: eBay seller curiodream100
Contributor: Theodore Gray
Acquired: 13 January, 2010
Text Updated: 13 January, 2010
Price: $50
Size: 10"
Composition: CuZnCH
|
|
|
|
|
|
|
  Azurite and Malachite. Azurite and Malachite.
Azurite and Malachite (Cu+23 (CO3)2 (OH)2 mon. ; Cu+22 (CO3) (OH)2 mon.), Brixlegg, Tyrol, Austria. Nice, from an historic alpine mine. 2,2x1,5x1 cm; 10 g with box.
Source: Simone Citon
Contributor: John Gray
Acquired: 13 January, 2010
Text Updated: 13 January, 2010
Price: Trade
Size: 1"
Composition: Cu3(CO3)2(OH)2+Cu2(CO3)(OH)2
|
|
|
|
|
|
|
Native copper nugget. (External Sample)
Copper, like gold, occurs fairly pure in nature, in lumps. Reader Jim O'Brien writes:As a woodworker and a resident of Michigan's "Copper Country", I find your Wooden Periodic Table most intriguing. Thanks for all your work in developing and posting the website.
Just to add another data point, the native copper specimens you show from the Harvard Natural History Museum almost certainly originated from the Lake Superior copper region and likely from the Calumet & Hecla mines on the Keweenaw Peninsula. They look familiar to me. I see kids selling them on the roadside to tourists all summer long.
Boston money financed the mines up here (Shaw, Quincy, Adams Paine, etc.). Alexander Agassiz, an avid collector and son of Louis (founder of the Museum), was curator there for many years and was appointed President of C&H just after the Civil War (http://www.famousamericans.net/alexanderagassiz/). Mining captains would compete for the best specimens, rewarding miners who could deliver the most spectacular examples from the deeps. Some mines up here go down over 9,000 ft.
Location: The Harvard Museum of Natural History
Photographed: 2 October, 2002
Size: 18
Purity: >90%
|
|
|
|
|
|
|
|
|
|
Birthday present for Oliver Sacks. (External Sample)
I went to New York with my six-year-old daughter Addie on somewhat of a whim, to attend Oliver Sacks' 70th birthday party. We had a great time: You can read all about it.
Of course I had to bring an element present, since Sacks is an element collector like myself (he came to visit my table about a year earlier). 70 (ytterbium) is kind of a boring element, so I decided to bring him some elements that add up to 70, aluminum (13), copper (29) and nickel (28), in the form of this little candy dish.
Location: Oliver Sacks' Office
Photographed: 9 July, 2003
Size: 8"
Purity: 33%
|
|
|
|
|
|
|
  Copper copy of iridium slab. (External Sample) Copper copy of iridium slab. (External Sample)
Look under iridium and you will find a picture of Oliver Sacks holding a 1.7-pound slab of iridium I helped him have made in New Jersey. This is a copy of that slab cast in copper years later, hopefully the first of several copies made in different metals. I have a silicone rubber mold I made of the original and over time hope to make more and better copies. This one is not very good.
Location: Oliver Sacks' Office
Photographed: 28 February, 2009
Text Updated: 1 March, 2009
Size: 2.5"
Purity: 99%
|
|
|
|
|
|
|
|
|
|
|
|